| Pages:
1
2
3 |
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
When finishing a liquid-liquid Iextraction gently swirl the sep. funnel to knock down last droplets from the walls.
When encountering emulsions gently heat the emulsion from the outside of the sep. funnel with a heat gun. The increased temperature increases the
molecular movements causing the emulsion to break up faster. (note that occasionally cooling is beneficial as some but not all solvents become (more)
immiscible below a certain point, see for instance the bahavior of MeCN-H2O. Sometimes solvents become less miscible by increasing the temperature (as
is described in Vogel's). I do not know how ternary systems behave but I had the feeling that the DCM-MeCN-H2O system gave better and faster
separations at or below 0C.
And finally for those ocd on purity, use the stopcock of a sep funnel to capture the thin film present at the interface and remember to remove the
bottom layer through the bottom and the top layer through the top (after you've drained the bottom layer obviously).
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I often use a reflux condenser above the distillation receiver, especially when distilling volatile chemicals. Like this: http://www.prepchem.com/synthesis-of-p-toluquinone/
I think this is fairly common.
[Edited on 14-9-2018 by JJay]
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Eyewash: it doesn't have to be a professional eye wash.
A squeeze bottle of saline from the contact lens section of your local store works wonders. But always have something to use as an eyewash, even if
it is just a bottle of water.
O-rings for thermometer adapters: Automotive O-rings that are suitable for E-85 and regular gas are made of viton. This is compatible with most
things except ketones, and even then they are cheap to replace, most automotive stores carry a variety. You can get teflon o-rings as well, they are
literally more expensive to ship than buy (US$ 1.30 for 5). A ground glass thermometer well never has o-ring problems and you can use regular cooking
oil as a heat transfer solution without worrying about contamination.
A better option for the use of teflon is to wrap the thermometer below the adapter and then pull it up to seal it and protect your 25 cent o-ring.
Lab apparel: Jeans and a long sleeve shirt are a minimum. Protect your arms. Wear boots not cheap shoes. Pretend like you are cooking and moving
around hot oil. A lot of lab chemicals aren't nearly as bad as hot oil but working like it is will save a lot of trouble later.
Fires: If you have a fire, you did something wrong. BUT, you should have appropriate materials to put out the fire you are working with. Baking soda
works better than water on most organics as it releases CO2 and doesn't spread the fire. Oil or sand work better on metal fires than
water. But you may need to extinguish the oil after you put out the metal.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Same here :'D Lab coat is not a piece of clothing, rather a line of defense, something to quickly remove and throw away when compromised with some
nasty chemical.
The material (cotton) is especially good in absorbing stuff.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Improved thermometer.
It's simply the TM-902C probe inside a thermometer adapter filled on bottom with thermal conductive paste and some glass wool and duct tapes to keep
it all in place. This improves response and accuracy of the readings.
Credit goes to ChemX YouTube channel.

|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
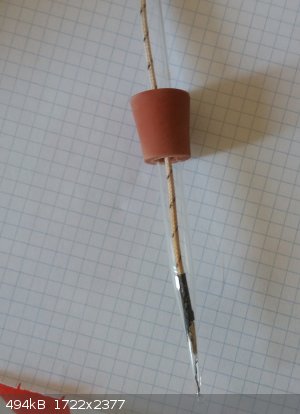
i did a similiar thing some time ago when i broke my only mercury thermometer, i used a pipette, melted the tip and inserted the K-therocouple to its
bottom, the glass there is thin so the heat capacity is small, the temperature reading is really fast upon changes in temperature. i think that with
all that conductive paste you have quite some heat capacity, less is better to get a fast response
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Quote: Originally posted by Ubya  | i did a similiar thing some time ago when i broke my only mercury thermometer, i used a pipette, melted the tip and inserted the K-therocouple to its
bottom, the glass there is thin so the heat capacity is small, the temperature reading is really fast upon changes in temperature. i think that with
all that conductive paste you have quite some heat capacity, less is better to get a fast response
|
Good idea thanks. I have lots of pasteur pipettes but unfortunately no way to melt them. I guess a butane torch lighter wont be hot enough right?
I needed an adapter instead of rubber stopper because sometimes I distill aggressive stuff and the rubber would degrade. But I guess something similar
can be done with an air inlet tube.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
One more tip is to have certain common chemicals in a freezer, especially brine, sulfuric acid and solvents like acetone.
Brine can be used to chill a solution below 0 °C, it's more effective than an ice bath and is always liquid so easy to recycle/reuse.
Pre-chilled sulfuric acid is practical for dilution - in most cases adding it to a reaction mixture is highly exothermic so the pre-chilling helps a
lot.
Chilled solvent (e.g. ammonia solution, acetone, ethanol etc.) is practical for washing products.
It also comes in handy to have distilled water frozen in ice cubes (amount can be weighed). This way you can pour hot stuff on it or quench a reaction
without having to set up an ice bath beforehand.
I have a portable 30L car fridge/freezer for lab use. It has a compressor and thermostat so you can even set temperature down to -20 °C.
...or and a microwave is probably the fastest way to heat up water. It saves lot of time.
[Edited on 15-9-2018 by nimgoldman]
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Use a glass stir rod to help pour liquids. Keep several scupulas on hand and have one specifically set aside for heating substances.
Check thrift stores for cheap equipment like compressors, pumps, glass containers, fridges, power supplies, heating elements, and even depression
glass.
Keep a UV light in your car for finding interesting minerals or depression glass.
Do reactions on a bulk scale if you are going to be using the product often. This includes making nitric acid, diethyl ether, or dry solvents. This
boosts yield as you lose less to mechanical losses.
Make stock 1 M solutions of test solutions and titrations and keep them in bottles with droppers. Its also very ascetically pleasing.
You can cut old aerosol cans and propane tanks open to use as reaction vessels or chambers.
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by nimgoldman  | Quote: Originally posted by Ubya  | i did a similiar thing some time ago when i broke my only mercury thermometer, i used a pipette, melted the tip and inserted the K-therocouple to its
bottom, the glass there is thin so the heat capacity is small, the temperature reading is really fast upon changes in temperature. i think that with
all that conductive paste you have quite some heat capacity, less is better to get a fast response
|
Good idea thanks. I have lots of pasteur pipettes but unfortunately no way to melt them. I guess a butane torch lighter wont be hot enough right?
I needed an adapter instead of rubber stopper because sometimes I distill aggressive stuff and the rubber would degrade. But I guess something similar
can be done with an air inlet tube. |
I just melted a Pasteur pipette over the gas burner on my stove, so I'm pretty sure almost any torch would do the trick.
I haven't had any luck melting PYREX that way though, but Pasteur pipettes are soda glass I think. (Sure does melt like it.)
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Cut some thick rubber tubing to cushion iron rings that hold sep funnels. You don't want a funnel breaking when full of fluids.
It always makes my teeth stand on edge when I see glass-on-iron in chem videos.
Phlogiston manufacturer/supplier.
For all your phlogiston needs.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Use sealed off pipettes or small test tubes to make ampoules to hold volatile or air/moisture sensitive compounds. Much cheaper than buying ampoules,
too.
Phlogiston manufacturer/supplier.
For all your phlogiston needs.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SWIM  |
I just melted a Pasteur pipette over the gas burner on my stove, so I'm pretty sure almost any torch would do the trick.
I haven't had any luck melting PYREX that way though, but Pasteur pipettes are soda glass I think. (Sure does melt like it.) |
I seal Pyrex and Kimble capillary tube tips with a stove flame. And on boro Pasteur pipettes you can seal both sides with this sort of flame (you will
need to thoroughly pre-heat your crimping tool for the broad side though).
Boro Pasteur pipettes can be sealed at the tip with a bic lighter, even!
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by happyfooddance  | Quote: Originally posted by SWIM  |
I just melted a Pasteur pipette over the gas burner on my stove, so I'm pretty sure almost any torch would do the trick.
I haven't had any luck melting PYREX that way though, but Pasteur pipettes are soda glass I think. (Sure does melt like it.) |
I seal Pyrex and Kimble capillary tube tips with a stove flame. And on boro Pasteur pipettes you can seal both sides with this sort of flame (you will
need to thoroughly pre-heat your crimping tool for the broad side though).
Boro Pasteur pipettes can be sealed at the tip with a bic lighter, even!
|
I have done some of the capillary tubes with a bic for shits and giggles but have never tried a boro glass. Interesting info 
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
the rubber "bumpers" on the magnet off old subwoofers make excellent RBF holders
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
I doubt many people are going to have that available but it would probably work well.
I'd bet most people have some old garden hose, vinyl tubing or even old inner-tube (car, tractor, bike, etc). I'd think that all of those would work
well. Failing that, I don't see why you couldn't use paper towels (fold it over a few times and put it between the glass and metal.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Pulloff the back of an old subwoofer, nice and "grippy" from being rubber
 
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
sodium_stearate
Hazard to Others
  
Posts: 255
Registered: 22-4-2011
Location: guard duty at the checkpoint
Member Is Offline
Mood: No mask.
|
|
That's One Less!!
Nice work! That is one less of those annoying
speakers that always go BOOM BOOM BOOM in the night
as some dweeb drives around in his ghetto blaster!
"Opportunity is missed by most people
because it is dressed in overalls and it
looks like work" T.A. Edison
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by morganbw  | Quote: Originally posted by happyfooddance  | Quote: Originally posted by SWIM  |
I just melted a Pasteur pipette over the gas burner on my stove, so I'm pretty sure almost any torch would do the trick.
I haven't had any luck melting PYREX that way though, but Pasteur pipettes are soda glass I think. (Sure does melt like it.) |
I seal Pyrex and Kimble capillary tube tips with a stove flame. And on boro Pasteur pipettes you can seal both sides with this sort of flame (you will
need to thoroughly pre-heat your crimping tool for the broad side though).
Boro Pasteur pipettes can be sealed at the tip with a bic lighter, even!
|
I have done some of the capillary tubes with a bic for shits and giggles but have never tried a boro glass. Interesting info  |
I have checked my big box'O Pasteur pipettes, and they are in fact boro, as happyfoodance's are.
I just assumed they weren't because they melt really, really easily.
Must just be the thinness and fine point that makes em melt so nice.
|
|
|
WangleSpong5000
Hazard to Others
  
Posts: 129
Registered: 3-11-2017
Location: Oz
Member Is Offline
Mood: Curious
|
|
Wear safety glasses fucking always, have a fire extinguiser on hand of the correct type, use boiling chips and never take your eyes off a
distillation, or at least keep an eye on it
Hyperbole be thy name
|
|
|
TriiodideFrog
Hazard to Others
  
Posts: 108
Registered: 27-9-2020
Member Is Offline
|
|
You can replace boiling chips with some acid-washed sand and always wear safety equipment. To be honest, you can attempt to extract some chemicals
from Over The Counter products, although the yield might not be as good.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | The O-ring size is important,
I have a screw-top thermometer adapter and a screw-top still head adapter,
as supplied with my cheap distillation kit.
I cannot use the thermometer supplied with the kit (c5.5mm dia.) as it will slip,
but my 'normal' thermometers (c6.5mm dia.) fit very well. |
I'm wondering if water glass can be used as filler/glue to just seal the thermometer
Into The thin screwtop neck.or if I can just melt the t
screwtop and thermometer into each other.i think it could be done without
overheating the thermometer.maybe a very thin Pyrex filler rod is needed.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by Mabus  | @Sulaiman In that case I shouldn't have written the article, since more than half the info is from the forum chemists and I haven't done it myself.
What I'm trying to say is that just because you done it yourself and it worked, doesn't mean it's the ideal path. For example, I see in plenty of YT
videos chemistry professors handling alkali metals with gloves, while in other places (my former university, this forum, etc.) we're always told to
handle alkali metals without gloves and use either tweezers or spatulas. If I followed my professors advice, I'd shouldn't use gloves, but had I
worked with the other professors, I should have. So which is which? Who do I trust? That's why I wrote the article, to pool all the tips from all
kinds of chemists with different experience and compare them.
[Edited on 7-4-2018 by Mabus] |
I think the guy who said to use gloves is right.
|
|
|
outer_limits
Hazard to Others
  
Posts: 139
Registered: 3-3-2020
Member Is Offline
Mood: hybridized
|
|
Quote: Originally posted by macckone  |
Fires: If you have a fire, you did something wrong. BUT, you should have appropriate materials to put out the fire you are working with.
|
One of the first things I bought for lab was 5kg CO2 fire extinguisher. And this my advice for everybody - do not try to save money on protective
equipment. It's better to wait for reagents than having your house burned.
Wear eye protection whatever you do when working with toxic/corrosive/irritant chemicals. Even if you don't expect any splash it may occur. I had once
that happened after opening the stopcock of sep funnel. Fortunately I use it almost always in fume hood and hood's sash saved my eyes from strongly
alkaline solution.
Cut you pH indicator papers to smaller pieces, there is no need to waste entire paper just for one pH check.
Always leave some space below the flask, so you could swap immediately from heating mantle to cold water bath and slow down the reaction.
If you break a plate or mug at home - do not throw it away . Shatter it to small pieces, these are great boiling stones.
Plastic wrap is good for sealing, not as flexible as parafilm but good enough.
If your lab is located in a dusted, dirty space - use plastic wrap to keep it clean.
If there is a moisture - keep your moisture-sensitive reagents in string bags.
Use teflon tape to seal bottles. Teflon tape can be used as an alternative to joint grease. And it's much cheaper than teflon sleeves available to buy
in laboratory equipment shops.
Separate your reagents regarding their compatibility - you don't want to keep strong acids with strong bases as well as oxidizers and reducers.
Keep the bottles as low as it's possible. If they fall down from the shelve there is a probability that they could survive without breaking.
[Edited on 27-10-2020 by outer_limits]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Fire extinguisher is an absolute mandatory. In some instances it can be replaced with ordinary water hose with for ex garden nozzle that can turn the
water into mist. Water mist has an impressive fire extinguishing properties, I've been in fire exercise where we had to put off burning 40'
containers. With fire hose, it needs only three bursts of water to suffocate the whole fire.
This requires you to have mains switch directly next to hose or an RCD installed for all the electric equipment to not get electrocuted. Volatile,
flammable solvent in for ex case of flask breakage will immediately create immersive flames that can burn down your entire building if you cannot put
it away in seconds. Even here I've read multiple occasions where people have broken flasks, beakers and stuff directly on their hotplates by some
reason - maybe they were just not properly annealed. CO2 can suffocate, though, so ventilation becomes a vital aspect if it has to be used. Powder
extinguisher is an option too.
Sometimes one should also just sit back and think what really cuts costs or trouble. I've concluded that just simply buying various items like
thermowells is much more convenient than playing around with all kinds of rubber, silicone or similar plugs.
And I mean buying, because they cost so little. 2€ for thermowell is just nothing. I've literally built another set of labware at my home including
all accessories for a few hundred euros.
I use lab jack and install it so I can at any time remove the heating bath completely and if necessary, switch into cooling bath. This is important
for quick heat control and can be life-saving in case of runaway.
I also want to keep every apparatus attached to a stand. I've installed permanent threaded rods in my fume hood. Once, over 10 years ago, when I was
starting chemistry, I actually crashed an entire distillation apparatus to the floor because lack of stands. Silly me. What surprised me though only
the condenser attachment broke and almost none of the process fluid was lost.
|
|
|
| Pages:
1
2
3 |