notoxicshit
Harmless

Posts: 37
Registered: 18-4-2016
Member Is Offline
Mood: No Mood
|
|
amino acid + O² = a-nitro acid + formaldehyde - CO² = nitroalkene
I want to research organic reduction methods, from nickel urushibara, over Al/Ga & Al/Cu to selfmade red-Al and orher hydrides.
For this I need substrates, and one particularly tricky thing are reductions which combine double bond and functional grp reduction.
To develop easily accessible combos of reduction reagents I need substrates to practise exactly that with.
On my list now is due: making an nitroalkene for this purpose
I have already settled on a target and, especially, a precursor.
So I want to utilize an amino acid and turn it into a nitropropene.
This is as much about getting there than being there, the journey is the reward as well and I'm all about using amino acids for synthetic purposes.
The precursor thus is alanine:
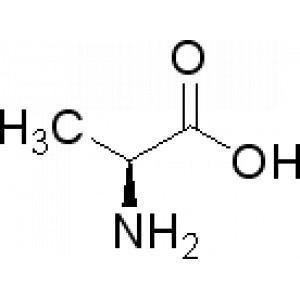
I want to discuss the easiest and most down-to-earth way to turn this into alpha-nitromethylethene, or 2-nitroprop-1-ene:

There are great many ways of doing so, but many would involve removing the amine groups' nitrogen and reattaching it as a nitro group nitrogen later,
which is not acceptable for me, as little as using some other precursor.
While its certainly possible to proceed via alaninol, using fancy reagents, I would much rather like to discuss a Knoevennagel type of reaction, which
could, in my pretty limited theoretical understanding, produce such a double-bonded nitroalkane.
What one could try is oxidizing the alanine to an alpha-nitro-acid, a type of compound I barely could find anything out about.
But I'm confident this could be done via DMDO or even simpler, so Id like to concentrate on where the insecurities lie:
The introduction of the missing alpha-methyl-carbon.
Lets assume we have oxidized alanine successfully to the nitrocarboxylate, could that one be subjected to a somewhat modified Knoevennagel
condensation?
(FYI: those are amine or better yet, aminoacetate-catalysed nitro & aldehyde coupling reactions)
Since the nitrocarboxylate is lacking only one carbon, I thought about condensing it with the shortest aldehyde, formaldehyde.
Condensing, the reaction would give off water like all Knoevennagels do (great for judging progress of rxn) and when I'm not mistaken, give me the
targeted nitropropene, but with one CO2 too much, which could be decarboxylated.
Major things to tick off:
1. Is this theoretically sound? Byproducts?
2. How to modify the knoevennagel to work with a nitrocarboxylate on one and such a volatile aldehyde on the other side?
3. How to check for purity/identity between the steps?
Minor:
A) how to best make that a-nitro-acid? Very hard to search about but DMDO might work and if it does, we can modify.
B) how to decarboxylate as the last step? No experience.
[Edited on 8-4-2018 by notoxicshit]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Could you use the aliphatic diazo compound route to replace the amino group with a chloro-group ie prepare 2-chloropropanoic acid and then react it
with a nitrite an decarboxylate to nitroethane followed by your condensation with formaldehyde. I am sure this route to nitroethane will have been
discussed somewhere on the internet (the Hive probably) if not on S. The conversion of alanine to chloropropanoic acid certainly has been discussed on
SM before. There is also a procedure in Vogel and elsewhere for the similar preparation of nitromethane from chloroacetic acid.
Just one thing with this route; formaldehyde sometimes condenses with two moles of counter compound in spite of the presence of two replaceable
hydrogens on the alpha carbon site. This might lead to a dinitropentane. A alternative byproduct might be 2-nitro-n-propanol in a similar fashion to
the formation of nitroethanol from nitromethane.
|
|
|
notoxicshit
Harmless

Posts: 37
Registered: 18-4-2016
Member Is Offline
Mood: No Mood
|
|
I first need to understand why you proposed an alternative. What thought lies behind that?
Like I said I'd much prefer to keep the nitrogen where it is, this is exactly what makes this kind of precursor so interesting: nature has put
everything in the right spot.
Unfortunately, if I were to try diazotization, I would have to make the commercially prohibited nitrite myself and also I prefer to leave out
halides, whenever I can.
If that was a viable pathway to nitroethane, I bet that we knew because there seem many people keen on it.
I want to make this atom efficient and if there's waste than it better be non-nasty.
I may check alternatives like this when the original fails.
I'd like to hear a judgement on this as it is. Doable?
Its just two steps with easy to get, cheap stuff.
Experiences with a formaldehyde knoevennagel?
Experiences with amino acid oxidation?
Ideas on procedural design, how to get the formaldehyde in there?
Im an experimenter, the opposite of an armchair chemist.
But it makes no sense to just start head over heels, when the theoretical odds may be staked against my unsuspecting ass.
[Edited on 9-4-2018 by notoxicshit]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
If you don't like my suggestion, no sweat.
May I politely suggest then than you UTFSE and do your own reading; there's over 100 years worth of paper on the subject of the oxidation of
alpha-amino acids so that should keep you busy for a year or so. Happy reading.
My suggestion was based on a the availability of published procedures. There is a fair body of work on the oxidation of peptides with
dimethyldioxirane as a method of investigating peptide structure but I'll leave you to read upon this topic
|
|
|
notoxicshit
Harmless

Posts: 37
Registered: 18-4-2016
Member Is Offline
Mood: No Mood
|
|
Its not that I dont like your suggestion, its that I just dont have the reagents.
As I said, I will try the alternative when the straigth way doesnt work - when the carboxy rest makes problems.
Thats why I asked for the purpose of that alternative - does a carboxy rest theoretically hinder that consensation?
You could also send me nitrite salt if thats OTC available to you. I'd pay you of course.
Still doubt if we wouldnt have heard from that. Interest in nitroethane is huge.
When theres reason to believe condensation without the carboxy rest works better then all power to thst suggestion
The oxidation is not expected to become a problem, that will work fine.
I'd mainly like to discuss how the condensation differs from a knoevennagel to PEA I had made before, using benzaldehyde and nitroethane (giving a
terminal nitroalkane that behaves somewhat different) and a n-butylacetate catalyst and no solvent besides a little IPA and minimal excess of GAA to
the n butylamine.
If I could use Paraformaldehyde instead etc.
Often people have experience in very similar cases and that would be wonderful.
[Edited on 9-4-2018 by notoxicshit]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Perhaps something in here might put you on the right track?
https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/fi...
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Amine to nitro conversion is stupidly difficult. Dimethyldioxirane is the only reagent that gets good yields. Tertiary amines are the exception;
iodine can be used in that case. But those are not Henry rxn substrates...
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
notoxicshit
Harmless

Posts: 37
Registered: 18-4-2016
Member Is Offline
Mood: No Mood
|
|
Yeah no problem really, I'm fine with DMDO since acetone and oxone are both cheap. Also not a problem in terms of the huge solvent volumes. Rotovape
off and reuse.
"those" are not for Henry? The tertiary, not the secondary amino acid?
Atara, why stop here? I've been waiting on you to chime in.
Is that condensation doable or will it suck badly?
How would you go about that?
All Im asking for is three to four competent people tell me if I am wasting time or if that idea might become a suceessful experiment?
[Edited on 10-4-2018 by notoxicshit]
|
|
|
notoxicshit
Harmless

Posts: 37
Registered: 18-4-2016
Member Is Offline
Mood: No Mood
|
|
What is it that is wrong with this thread?
The point is:
I really am motivated to experiment on a pretty serious basis (= contribution to the community) and all I need before starting a project is a brief
overview by my valued peers to make sure I dont waste time, material and environment.
I dont need spoonfeeding and I have a specific point, which is unclear:
Does a henry type condensation - using formaldehyde and a alanine-derived alpha-nitro-acid under base catalysis - theoretically work well enough or is
this the scheme's bottleneck?
The most useful answer so far was along the lines of "you get no answers cause its a bad scheme, duh.".
Can you guys top that?
[Edited on 23-4-2018 by notoxicshit]
|
|
|
Texium
|
Thread Moved
24-4-2018 at 04:31 |
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
First of all, good luck turning an amino acid into a whatever you seem to believe you'll be able to turn it into. There would almost certainly be
rearrangements or polymerizations or something. Also, when chemists talk about "nitroalkenes" or "nitroolefins", they're usually referring to α-β
nitroalkenes. These are formed via the Henry reaction mainly, but can also be formed by directly reacting nitric oxide with alkenes, although this is
not a reaction I've personally done. I believe that one main reason that these are commonly used is because they tend to be quite stable, and so can
be isolated and purified fairly easily. The "alanin-derived nitro acid" is where you go wrong, since it's hard to imagine you (or anyone) succeed at
synthesizing something like that.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I concur with Melgar; I did find a paper specifically about the oxidation of alpha-amino acids to the nitro analog, so yes it can be done BUT it
requires fluorine (yes the free element) in moist acetonitrile; the active species is alledgedly a hypofluorite-acetonitrile complex. The yields are
claimed to be pretty good but given the required conditions not really amateur friendly. I also found a paper of the oxidation of amino acids and
peptides with dimethyl dioxirane as a means of characterising the amino acid fragments but nitro-acids were not the products of this reaction.
Since I found both of these papers using google in about 10 minutes. I leave you to hunt them down if you are really that interested but it looks like
a hopeless route.
I also came across papers for the preparation of alpha-nitroalaphatic acid by various process. I would appear that the acids are fairly stable in
solutions containing 2 equivalences of base but the monobasic salts and free acid tend to decompose all in part by the loss of CO2.
It's amazing what you can find out if you are prepared to do a bit of work!
|
|
|