RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
Where does heat go in a battery?
Or in a cell. Why these cells heat up? What if I am supposed to use battery as heat. When i connect it to some wire which is supposed to heat up,
battery will heat up as well? Where is more heat released? Why? What is theory of heat? Is heat higher in higher or lower resistance wire?
I thought about this problem for last 10 years, and could never figure it out. The simplest view is to simply view whole battery as chemical reaction
and forget about electricity, then it will release heat called heat of reaction. But could never imagine it as two separate parts, one is battery,
another is object which is pluggen into this battery. If I am supposed to use battery as heater, seems only sane is to use whole battery as heater. So
complicated...
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
The reaction is exothermic. Ideally the energy released is all electrical but this is never the case in reality. Some heat is always produced. The
heat dissipates in the same way that heat usually dissipates -- by warming up the things around it.
In a situation where the rate of heat energy produced is high enough and the battery is sufficiently insulated, it is possible for a series of runaway
reactions to occur -- that is, your phone catches on fire and you are not allowed to fly on aeroplanes.
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
I know that, but don't know what determines where this energy will be released. In case of using external resistance heating, we can look at it as two
heats. What is telling the battery or cell: "hey, you release your heat or energy outside after passing wires that connect you to device, and you
release that heat inside battery". How much percent inside, how much outside? Is it depending on resistance of external device or what?
Looking at short circuited battery alone is simple. All energy is released in battery. But how wires divert this energy to some external device? Who
is responsible for energy or heat? Electrons or ions? Electrolyte or electrodes? What gives energy? What diverts energy? What is inert?
[Edited on 10-4-2018 by RawWork]
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I doubt there is a simple answer to that question. If I roll a ping-pong ball down the stairs, some of the gravitational energy goes to kinetic, some
to producing sound, some to heat and some to causing physical deformation or wear on the ball. It is easier to determine these proportions
empirically than it is to predict energy distribution from theory.
In a battery there is internal resistance (which is itself a simplification of a number of different processes.) There are also competing reactions.
IOW there are different ways that heat may be produced inside the battery. The exact proportion of these will be highly dependent on geometry, the
distribution of materials including reaction products in the battery, the dynamic nature of the electrical load and all manner of things. Ultimately
your best approach is to test the battery under a range of conditions and assign to each experiment an efficiency based on the proportion of the
available energy that ends up where you want it (ie, electroical). You really don't worry about heat unless you start having temperature problems
with your battery design.
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
There is an electrical engineering approach:
I'd ignore the chemistry entirely and consider the battery as
a fixed voltage source, Vb (due to chemistry)
with an internal series resistance, Rb (due mainly to physical resistance, only partly electrolyte resistance)
If you measure the voltage across the battery terminals,
with negligible current drawn from the battery, the voltage will be Vb
If you short-circuit the battery then the current will be Vb/Rb (Ohms Law)
Most batteries are not designed for a short circuit load so will fail, often dramatically.
If you put a resistor (Rx) across the battery as a load,
then the total circuit resistance will be Rb + Rx
so the current (Ib) = Vb/(Rb + Rx)
The POWER dissipated in the load (Rx) will be Rx x Ib2 watts,
the power dissipated within the battery will be Rb x Ib2 watts
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
That's well and good Sulaiman. But the OP was asking about the chemical factors that contribute to the heating side of the system. Ignoring the
chemistry does not answer that (although what you have detailed probably gives a reasonable practical answer to the energy distribution).
I am going to take a stab here and suggest that Rb is probably not constant but varies with current and also with the life of the battery.
That said, I doubt anyone could present a better answer than what you have supplied without getting into some specific battery designs and some pretty
sophisticated thermodynamics and computer modelling.
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Although there may be some heat due to exothermic reactions I suspect that it is negligible compared to electrical heating due to I2.R
because neither charging nor discharging has ever produced a cold cell in my experience.
As primarily an electrical engineer I would also not bother with any simulations,
cell voltage vs. time with load as a parameter is basic datasheet graphical lookup stuff.
EDIT: Generally speaking, electrical engineering ignores chemistry until it fails 
Batteries seem a good example of 1% inspiration and 99% perspiration,
new battery chemistry is always being researched,
but the majority of effort is in materials and manufacturing technologies,
the devil is in the detail. (££££££££££)
[Edited on 10-4-2018 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
Of course, practically it's easy to calculate efficiency. Especially in case of heater device. Then efficiency (%) is energy released in device vs
energy released on batery * 100. Much simpler than any theory.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Electrical based heat comes about as the electrons knock into atoms, transferring some of their energy to kinetic vibrations in the atoms. That’s
basically the only prominent rule which dictates it. Like Sulaiman said, P=I^2*R in basic terms:
Current shows how many electrons are travelling through the material, hence how many electrons are colliding with atoms.
Resistance shows how difficult it is for atoms to get through the material because of how often they collide with atoms.
Hence increasing either/both of these means more heat is released during the operation of a cell, and efficiency is decreased.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
When you use the word 'Battery' it implies a number of cells with any required electrical connections and physical packaging.
If you only want the chemistry part then it would be better to refer to an 'electrochemical cell'
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
Well, the constant is the same. Efficiency doesn't change. Also some people never heard about cells. Battery is common name that villagers, rednecks,
kids, retards, gypsies and all people have heard of. In case of AA 1.5 V battery it's really a cell and a battery at the same time, LOL. Everybody
calls that cell a "battery". "Cell" sounds too scientific. Actually in Bosnian/Croatian/Serbian language old people don't even know what is a battery.
They call flashlight a "battery" and battery a "filament". Weird 
[Edited on 10-4-2018 by RawWork]
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
LearnedAmateur
I'm looking for clarity, not a fight,
Quote: Originally posted by LearnedAmateur  | | Electrical based heat comes about as the electrons knock into atoms, transferring some of their energy to kinetic vibrations in the atoms. That’s
basically the only prominent rule which dictates it. Like Sulaiman said, P=I^2*R in basic terms |
What you wrote is what I believe,
then I thought "why is heat proportional to I2 in this physical model ?"
Answer: (I assume)
The electrons are being accelerated by, hence gaining energy from, the electric field gradient.
So P = V x I is a better representation of heat generation than P = I2 x. R
Of course they are interchangeable as V = I x R
most electrical units are derived anyway so obfuscate physical understanding.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
I know what parameter increases heat or energy. But more care about where? P=I^2*R is more like optical illusion because one parameter is hidden
inside another and is opposite. That equation tells us that power is higher if resistance is higher and if current is higher. But we all know that
current is lower if resistance is higher. More practical to use U and R. So P=U2/R.
I am still curious where is this power, resistance and current? In a cell, in a wire from cell to device being powered, or in device being powered.
It's important where how resistant wire will cause what?
Many people say that heating is higher if resistance is higher. But equation shows opposite. That's what worries me.
[Edited on 10-4-2018 by RawWork]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
You may also wish to examine some reasons put forward as to why batteries seemingly unintentionally overheat! See https://www.techradar.com/news/why-lithium-ion-batteries-cat... .
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
I can imagine how wires between device and battery heat up. The more electrons collide in wire, that is the higher resistance is the hotter the wire,
the more losses. It's still weird tough. It means that the higher resistance is, aka the lower the current is, the higher the power or the heating.
But we all know that the higher the current is the more the heating or the power. I know that practically larger (wider) wire will reduce this loss.
But theoretically can't figure it out.
Not to talk about powered device losses, especially if it's wire alone.
It looks like in case of wire between battery and device, losses or everything is shared.
[Edited on 10-4-2018 by RawWork]
|
|
|
Twospoons
International Hazard
    
Posts: 1280
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Ok, so consider a hypothetical battery of 12V, with an internal resistance of 100 ohms, modeled below as voltage source V1, and resistance Rb. This is
a terrible battery, but its just to illustrate where the power goes.
Connected to this is a wire with resistance Rw.
As we vary the resistance of Rw from 10 ohms to 600 ohms you can see the power dissipated in Rb and in Rw varies. See graph. I've also included the
current flowing in the wire.
So you can see the location the power is dissipated is dependent on the relationship between the external load resistance and the internal battery
resistance. Does this answer your question?
One interesting point to note: the maximum power in the wire occurs when it has the same resistance as the battery. I've made the horizontal axis in
the graph logarithmic, as it highlights this feature. This is an important principle in electronics known as the maximum power transfer theorem.
I apologise for not labeling the vertical axes - Google sheets doesn't seem to let you have independent labels for left and right axes (they are power
and current respectively)
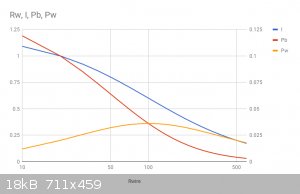 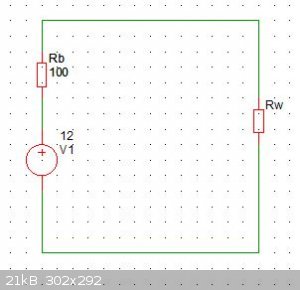
[Edited on 10-4-2018 by Twospoons]
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Quote: Originally posted by RawWork  | | It's still weird tough. It means that the higher resistance is, aka the lower the current is, the higher the power or the heating.
|
This can be demonstrated via the equation V=IR, or rearranged to form I=V/R, showing that current and resistance are inversely proportional.
Additionally, V can be substituted by P/I (from P=IV), where the equation becomes I=(PR)/I, rearranging to give P=I2R - dissipated power
has a proportional relationship with both the square of the current and resistance! Gotta love applied mathematics.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
Twospoons:
The left picture is unclear. What is changing horizontally? It says Rwire, but vertically it says Rw. So you made a typo in vertical, there should not
be Rw? I will try to figure out these pictures over next few hours... There's also apps like Electronics Workbench or NI Circuit Design Suite which I
will try to simulate such pictures... It will take time tough.
LearnedAmateur: Yeah, it's interesting how everything can be reverse engineered. Or everything can be made from everything.
[Edited on 10-4-2018 by RawWork]
|
|
|
Twospoons
International Hazard
    
Posts: 1280
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
No thats not the vertical label, thats the chart title. Google sheets isn't a great tool sometimes.
To be clear: the left vertical axis is power in watts, the right vertical axis is current in amps and the horizontal axis is Rw, the wire resistance,
in Ohms
Here: I've managed to clean it up for you.
[Edited on 10-4-2018 by Twospoons]
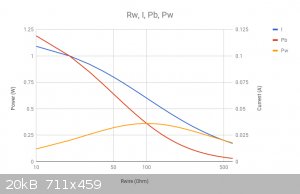
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
RawWork
Hazard to Others
  
Posts: 167
Registered: 10-2-2018
Member Is Offline
|
|
Aha, got it now! That makes sense.
|
|
|