| Pages:
1
2 |
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
yes ...
| Quote: |
So you expect the air and sulphuric acid/water vapour to flow in at the top at the same time that water vapour flows out of the top. Why would that
happen with the air flow and a condenser that must be cooler than the vapour or none of it will condense?
|
Because sulphuric acid condenses at a temperature well above the boiling point of water. The condenser is held at around 100C .... very strong
sulfuric acid *will* condense at that temperature, but water does not condense. So, the principle I'm using is the same as a reflux vigereux colum
... a hotter part of fractioning column has stronger sulfuric condensing, while a cooler part has weaker sulfuric condensing.
| Quote: |
I don't think it will work.
|
I already know that the heated condenser does work. I distilled over 200mL of rooto sulfuric acid using the set-up and a silicon carbide ball bearing
as a "glass" bead. Unfortunately, some of the carbon was etched... but the impurities in rooto did not make it into the collection flask. There
are probably more efficient ways (and complicated) to design a still, by moving the vigereux column after the collection bottle -- with some kind of
reflux trap to route all the reflux back to the boiler flask. But, I haven't figured that out yet.
| Quote: |
But try it with glass wool. Wash the pump out quickly at the end and do report back.
|
I do not have glass wool to test the cold trap after the collection bottle, but I have ordered it. There are going to be several days (possibly
weeks) between now and any tests I can do. Glass wool can still leak as there is going to be a scrunched up spot where the wrapped wool comes back on
itself. Holes along the seam are inevitable. But, I will still try it.
The idea with sand is an interesting possibility. However, isn't regular sand impure silica? It seems to me that reflux from a sand trap would have
unknown contaminants in it. The only place I could put a sand trap is after the vacuum tap ... and that's no different than using a neutralizing
chemical... So, it seems like a waste of effort and chemicals when unfired glass frit seems easy to make using a pin mill.
[Edited on 24-4-2018 by semiconductive]
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
I occasionally make a frit for some glassware applications, but the commercially made ones are usually the most economical way to go. If you want a
longer section of frit than the standard thin discs then my technique has some merit.
Getting a frit to work is quite a process, involving many steps and well calibrated equipment. Such as a furnace and a mold that can be inserted and
withdrawn with precise timing. Even when you have a frit, getting them sealed glass is another headache, it takes a good glassblower with considerable
experience to get it right without cracking on you, and that's with a good reliable brand of frit with good QC.
Glass wool and bits of crushed glass or beads as packing is the way to go here.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
| Quote: | Quote: Originally posted by semiconductive  | ...
| Quote: |
So you expect the air and sulphuric acid/water vapour to flow in at the top at the same time that water vapour flows out of the top. Why would that
happen with the air flow and a condenser that must be cooler than the vapour or none of it will condense?
|
Because sulphuric acid condenses at a temperature well above the boiling point of water. The condenser is held at around 100C .... very strong
sulfuric acid *will* condense at that temperature, but water does not condense. So, the principle I'm using is the same as a reflux vigereux colum
... a hotter part of fractioning column has stronger sulfuric condensing, while a cooler part has weaker sulfuric condensing.
|
We can agree on the temperature at which different strengths of sulfuric acid condense.
You did not answer my question directly: Do you expect the air and sulphuric acid/water vapour to flow in to the top of the column
at the same time that vapour with less sulphuric acid flows out of the top of the column?
You have already tried this set up and it worked? |
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
A fter looking back through the whole thread?
Please do report on an actual experiment.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by wg48  |
You did not answer my question directly: Do you expect the air and sulphuric acid/water vapour to flow in to the top of the column
at the same time that vapour with less sulphuric acid flows out of the top of the column?
You have already tried this set up and it worked? |
yes, it works. I said I already did an experiment -- but NOT with a trap before the vacuum takeoff and glass frit or floss, but only with a silicon
carbide bearing where the "red" mark is in the original diagram.
I have two experimental designs, the first experiment is already posted on sciencemadness.com with photos. The second is better, but I don't have
photos.
In my second experiment, the allihn condensor is oriented vertically.
There are swirls near the top of the condenser where the cooler air from the vigerux column hits the warmer air from the condenser. It's a bit like a
tornado, but very slow. The cooler air appears to go down the center of the allihn condenser, while the warmer air wants to go up the sides. As the
cool air warms up, I think it must expand and hit the sides of the (allihnn) condenser. That causes water/acid droplets form on the glass. Again, the
allihn condenser is vertical... but even if it were at an an angle, I still think it would work. The cooler air would sink to the bottom, while the
hotter air would move up to the top of the condenser. My first experiment used an angled allihn condensor with towels for insulation.
Why are you so concerned that it wouldn't work ?
Are you imagining that the air is moving fast?
There are photos in another thread of my first experiment (link below). The insulation has been removed from the horizontal Allihn condensor. eg: so
you could see the condenser in the picture and not a towel. I didn't know how to use the allihn condensor at the time, so it wasn't hooked up
vertically yet. Even so, the 150C boiler temperature kept the top portion of the condensor hot since I had no water flow in the cooling jacket. The
allihn condensor was effectively insulated by the jacket with no water in it. I think my thermometer measured 80C when I stuck it inside the exhaust
port of the condensor near the top in the shown experiment. After wrapping the connector and top of the tube with a towel (not shown) the temperature
went up. The bottom half of the allihn condensor tube formed water/acid droplets while the top half stayed dry. An angled allihn condenser is NOT as
effective (convection current wise) as one mounted vertically; But it still has a certain amount of convection currents happening when sufficiently
insulated. The strength of the acid in the alliihn condenser is enough to dehydrate cotton. It's not at all "weak" acid. The water at the top of
the vigerux column, however, will not dehydrate cotton.
So, I clearly did answer your question @ wg48 ... you're just having a hard time believing me ?
( And superadministrator, Burt, too? or is that just good advice? )
Look at the following link, this is an earlier example before I knew how to use the allihn condensor properly: Notice the washcloth that got dripped
on, and the paper envelope, have both been turned to carbon on the drip spots.
https://www.sciencemadness.org/whisper/viewthread.php?tid=79...
The fish tank pump is the blue one on top of the oscilloscope. I took the insulating towels off the allihn condensor to take the photos. The silicon
carbide bearing is clearly visible in the second photo. I didn't have a third vacuum take off valve at the time, so I used a sepratory funnel and a
marble with a bit of water as a sulfuric acid trap.
The vertical allihn condensor design for the second experiment is hardly more sophisticated than the first experiment I show in that photo, the only
differences are a hot air soldering iron tip is preheating air forced into the bottom of the allihn condenser. I also figured out how to make plaster
of paris + pearlite insulator which doesn't get ruined by acid like towels do. So insulating jackets are acid tolerant. And I have a bicarbonate
soda trap before the fishtank air pump's inlet, where I used marine grade epoxy to hook a pipe over the hole where the pump sucks air. So my fishtank
pump now has an inlet and outlet pipe, and an adaptor replaces the sepratory funnel so I can return air to the fishtank pump.
To get a feel for the airflow, which seems to be the mental issue people are having....
The cross section of 24/40 glassware is over 16x the area of fish-tank air hose. If you put a fish tank air hose from a pump for 2 to 5 gallon fish
tank into a steam from a pan or dust cloud from chalk, you can easily see yourself that the air does not move very fast and that the cloud will
"swirl" around the air from a 1/4 inch hose. In 24/40 glassware, the air could only be moving 1/16th the speed of what's in the hose -- and that's
divided in half because more than half goes up the vigereux. There is plenty of room for convection air currents to form as long as there are
temperature differentials of 10 degrees or so.
Again, when I don't put a glass bead inside the top chamber of the vacuum take off adapter (red color), then the cooler air makes it to the bottom of
the allihn condenser (300mm long) still laden with sulfuric fumes. So, it's quite possible the experiment wouldn't work if I set the system up even
slightly differently.
Everything I am telling you is obvious, and you can repeat the experiment yourself.
I don't know if the information people are giving me is accurate, but I do know what I have done. I will be happy to post mail tracking on the stuff I
ordered, to prove I'm not yanking your chains. Your time might be better spent finding out if the guy from argentina is really giving me straight
answers that are useful. People seem divided on whether he's given me enough information to reliably make a frit filter ; and I'm not sure either.
I get real tired of having my integrity attacked; my first thread on this site got moved to detritus because of judgmental suspicions, and the day
will come where the whole world will know the person who judged me was very wrong. I'm still very sore about that. I spend more time defending
myself than doing experiments when people act like Sir Isaac newton who set science back 300 years. He was a know-it-all, and was "dubious" about
someone else's experiment. When certain people come into power -- their intolerance is a problem.
[Edited on 25-4-2018 by semiconductive]
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by semiconductive  |
I get real tired of having my integrity attacked; my first thread on this site got moved to detritus because of judgmental suspicions, and the day
will come where the whole world will know the person who judged me was very wrong. I'm still very sore about that. I spend more time defending
myself than doing experiments when people act like Sir Isaac newton who set science back 300 years. He was a know-it-all, and was "dubious" about
someone else's experiment. When certain people come into power -- their intolerance is a problem.
[Edited on 25-4-2018 by semiconductive] |
I apologise if my use of the word 'dubious' offended you when I wrote
"I'm dubious of the functionality of the device"
I used the word meaning 'doubtful' - of the device - not your integrity.
You have proposed a completely novel setup operating on principles not completely/clearly understood by me,
and I suspect that your frit will 'choke'
so what is wrong in warning you of problems before you encounter them ?
Reading the posts above it seems to me that you equate criticising the concept with criticism of yourself,
that is just your ego causing you trouble.
You may do better if you ask why there is doubt rather than get upset about it 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I confess too that I am not fully folowing the process that you are suggesting. But I have not read every post in detail.
I would advise listening to chemetix. He does scientific glassblowing for a living and knows what he is talking about.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by j_sum1  | I confess too that I am not fully folowing the process that you are suggesting. But I have not read every post in detail.
I would advise listening to chemetix. He does scientific glassblowing for a living and knows what he is talking about. |
Thanks, I appreciate you seconding chemtex.
That's very helpful.
@i_sum1, @chemtex
I ordered the glass floss, yesterday. Borosilicate floss is cheap, but I live in the country and mail order of the lower cost stuff takes time. I'll
post pics when I try that method but be patient, it will likely be weeks before stuff arrives.
Sorry, my posts are so long. I never learned to condense ideas without making them too confusing. The more frustrated I am, the more verbose I get.
Hang in there chemtix, I'd love to pick your brains a little.
I think I need to show how advice in this thread compares to my budget reality ... but I want to emphasize that I don't want to try only the cheapest
solution; I'm wanting the "middle" road with reasonable chances of success and lower costs/even if longer time.
When it comes to buying glass frit discs off the shelf, China is generally cheaper than American made. But buying pre-made frits doesn't seem
practical for most amateurs. The lowest ebay/china introductory price is still $89.
https://www.ebay.com/itm/10mm-150mm-Sintered-Glass-Discs-Fri...
I can do a lot of home-brew experiments for $89.
I've done lots of searches ... and there *are* random sized frit discs sold by individuals (used) for $8, or teflon gaskted frits for buchner funnels
at around $25 each; but, basically, glass frits are custom made/modified things for specific applications. I can't buy a generic disc for the inside
lip of 24/40 adapters with a hole in the center for a drip tube.
$89 is a lot of money to buy a generic frit in order experimentally drill it ... when I don't even know what drill will work reliably.
Chemtex didn't offer to make a disc for me, So I assume that his earning a living prefers not to make frit disks. eg: He would have to charge a lot
because it's a "pain" to do, and there are other unspoken snags and price raisers like bulk purchasing prices.
I kind of figured that was the case when I started the thread; That's why I'd like to ask for advice, again... Let me re-iterate my capabilities
which are probably sufficient, if the right professional would guide me.
I have several computer based temperature controllers and kilns. Most amaateurs don't ... but they aren't hard to make. I can make them.
One example of my kilns is the alumina cement lined toaster overn in the background of the distillation apparatus photos. (left side, black toaster
oven)
https://www.sciencemadness.org/whisper/viewthread.php?tid=79...
That kiln tops out at 500C, but (@chemitix) I can (and have) made kilns for less than $25 that go to 900C at programmable ramp rates and times. I
don't mind building a kiln to try a sintering process, *IFF* I am given good instructions / decent estimates of temperature profiles vs. time. eg:
just close is fine. But I also don't want to spend a lot of time making something for a process that has little or no hope of succeeding or being
repeated by other amateurs. That's why I want an experts advice. I have fired clay ceramics and am familiar with quartz inversion points, pre-heating,
soaking, tempering processes, and glazes. So, I will be a very quick learner of anything you have to offer.
I do have alumina hydrate, sodium silicate, boric acid, and borax, with which to make borosilicate glass from scratch; These are all chemicals that
amaters can easily get or make from houshold/hardware store chemicals. If I had some guidance, I would willingly spend the time to refine a
practical, non-proprietary borosilicate glass formula and share the results.
I think generic borosilicate 3.3 glass AKA: *non-alkaline* borosilicate glass is about
81.1% SiO2
12.5% B2O3
4.2% Na2O
2.2% Al2O3
I'm pretty sure the correct oxides will form if I just maintain the same ratios of Silicon, Boron, Sodium, and Aluminum in precurser chemicals and
ignore the hydroxides and oxides. I think that making from scratch would be better than buying random borosilicate glass that might turn out to be 36
or 41 expansion rates, etc.
Whatever process I come up with, at least it would be documented and repeatable by other amateurs. eg: with less russian roulette effects due to
salesmen mistakes and brand loyalty or accidentally buying the wrong glass to make frit from.
Do you have any expertise with the glass making itself, know someone who does, or are you forced to buy glass frit in bulk from reliable suppliers at
non-amateur level prices?
[Edited on 25-4-2018 by semiconductive]
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
| Quote: |
I apologise if my use of the word 'dubious' offended you when I wrote
"I'm dubious of the functionality of the device"
I used the word meaning 'doubtful' - of the device - not your integrity.
|
Thank you, I appreciate that.
Be aware that others have, and will, see such remarks as attacks on my credibility.
| Quote: |
You have proposed a completely novel setup operating on principles not completely/clearly understood by me,
and I suspect that your frit will 'choke'
so what is wrong in warning you of problems before you encounter them ?
|
| Quote: |
that is just your ego causing you trouble.
|
That's not tactful; you clearly did not read my posts carefully and are shifting blame.
You primary (repeated) criticism was clearly not about the frit, but about air-flow at the top of a condenser where no frit exists.
What is wrong with warning me, is that you had no real evidence; you summarized what I said wrongly, and other people will assume you are correct
because most people are lazy and don't read carefully esp. in a long thread. So if I don't respond, your slap at my ego will go unchallenged and
rewrite history in most people's minds. Smart people are easy to attack on sciencemadness forums. You have (intentionally or not) also set me up to
be attacked by other posters. The EXACT same thing happened in the first thread I made.
I'm an electrical engineer, well above average but didn't graduate cum laude -- I have designed things like electric kilns, hydroponic controls, and
small supercomputer clusters for making satellite boards. There are other engineers who make bigger supercomputers than me -- I'm not the best, just
because I have one skill. But, people obiously like to attack and discredit any intelligent person who gives background information. The more
intelligent a person is, the more others assume they are an egotistical bastard.
I'm an easy hit.
The supermoderator, Burt, has a nice checklist of things to do before criticizing someone's idea. It's at the end of each of his posts as a signature
right now. It's written by another smart person. If you still don't "Get" it, search for my first thread ever, on scimadness. See how one person
saying "dubious" or "doubtful" totally derailed a thread and got my question condemned. It cost me a lot of wasted time and frustration. I spent a
lot of work, and one comment from a suspicious person tore it all down.
I have been told by other, kind, members that there is a troll on scimadness who posts theoretical stuff that isn't true; Many members are
hypersensitive to anything that looks suspicious ... therefore when anyone says my idea is "dubious","doubtful", etc. they automatically encourage
other members to assume the "troll" is me. I've been there, done that, got the T shirt, and don't want to see it again.
If the reason I want to make a frit filter helps you make positive suggestions about how I might make the filter not "choke" that would be good ...
but I don't see how your warning is helpful in any way in 1) encouraging or teaching me (and others) how to make frit filters. 2) in making me more
likely to succeed in what I'm doing.
Even if the frit filter doesn't work well in one particular application ... having a thread which teaches how to make a generic frit filter would be
useful to other amateurs. So, your warning only has the effect of discouraging me from even trying to do something that will help others, as well. I
generally try to learn skills that are useful in many ways, and not just in one experiment.
[Edited on 25-4-2018 by semiconductive]
[Edited on 25-4-2018 by semiconductive]
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
I can make a frit for you, I just thought you were involved in the challenge of trying to make your own, and why not sometimes, but it's a complex
task and I didn't want you chasing a goal that will sink more time and money than it's worth. Just for interests sake I've supplied a pic of a typical
frit that was from a reputable supplier, cleaned and prepped well, tooled nicely, moon ascendant in Sagittarius and still failed.
Give me a drawing with dimensions and your ideas about the design and I'll give you a quote.
I understand a bit about your design, and it seems like a mist trap to me, if I'm wrong please correct me but the thread is a little messy to follow.

|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by highpower48  | | Doesn't Deschem offer to make custom glassware. Not sure but that might be easiest and cheapest way to go. If you don't mind Chinese glassware.
|
Your idea appears to be spot on, thanks. 
Deschem just replied to my message on e-bay, they say they can make the whole adapter for $15. So, now I'll try ordering and see if they are
actually able to do it.
[Edited on 27-4-2018 by semiconductive]
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by Chemetix  | | I can make a frit for you, I just thought you were involved in the challenge of trying to make your own, and why not sometimes, but it's a complex
task and I didn't want you chasing a goal that will sink more time and money than it's worth. |
I do want the knowledge; but it's for a goal, and not just for knowledge's sake.
There are some glassware I'm not able to buy, and there are no general purpose glassware that can be used to make it easily. I'm not familiar with
costs of custom glassware, and any stock items in the U.S. market that are even slightly unusual seem to sell for astronomical prices.
For example; No seller advertises an adapter for European standard to American joints, they just adapt "sizes". I bought a European 24/40 to 19/23
adapters and the 19/23 joint went way too far into an american Pyrex flask. Pyrex measures the fitting diameter halfway down the fitting, but
European glassmakes measure fittings as the top.
In theory, I can just "cut" off the extra length on a European 19/xx adapter that penetrates too deeply ... but in reality, scoring and snaping ended
up breaking the joint in places not scored.
I made a ceramic mold from a pyrex 19/23 plug; and if I had frit, I could fix the joint. But, I'm just shy of the knowledge needed to repair it.
It's that kind of thing that keeps happening as I'm an amateur, that I'd like to be able to repair.
| Quote: | Just for interests sake I've supplied a pic of a typical frit that was from a reputable supplier, cleaned and prepped well, tooled nicely, moon
ascendant in Sagittarius and still failed.
Give me a drawing with dimensions and your ideas about the design and I'll give you a quote.
I understand a bit about your design, and it seems like a mist trap to me, if I'm wrong please correct me but the thread is a little messy to follow.
|
More or less, yes; The two frits are to stop mist and fumes. The inlet frit will probably also condense a minor amount of gasses that are hotter
than the frit; eg: gasses from the heated condenser are not homogeneous in temperature or composition.
There ought to be no mist inside the collection flask (after the condenser) because the top frit blocks the condenser mists. I expect that only
fumes will exist in the collection flask. The lower frit that protects the vacuum take off, then, should only be exposed to low concentrations of
strong acid fumes.
That's why I only plan to put a hydrophobic coating on the lower frit. Fumes of sulfuric acid do not like to go through silicone membranes, but air
does
I've got to go out, tonight, so I'll make some detailed drawings tomorrow and measure the dimensions of the adapters I have. I basically have three
ideas; but they are just variations on the same theme.
If you're able to make non-standard glassware at reasonable prices, there are possibilities that I'd like to ask about. I've been focusing on what
can be done by making minimal changes to standard off the shelf items; but as I learn, I've begun to realize that standard stuff has some serious
drawbacks.
[Edited on 27-4-2018 by semiconductive]
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by Dr.Bob  | | I have some various 24/40 m/f jointed tubes with a frit in the middle. Not perfect, but might work. I'll look for anything like what you posted,
but I might have something close. |
@Dr.Bob
I didn't mean to ignore your post, I just missed it. I haven't seen Eurpean/Chineese versions of a fritted straight joint for sale, and I have been
looking for them. I've only found mist blockers with straight glass tubing.
Are the joints you have European standard (24mm measured at top of joint, not middle)?
And, if so, how much are you willing to part with them for.... ?
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
After measuring the adapters, I noticed a problem. The places where the frits go are after a narrowing in the glass. I'm not sure how I can get the
frits into an existing adapter. I think the whole adapter may need to be made as a unit.
But, I'll post the design ... anyway ... in case you have any interest in bidding a complete part. If Dr. Bob's part would work, I may be able to get
away with an adapter that just has the lower frit.
To explain how I got the frit thicknesses:
Here's a chart I made from the ROBU(Tm) data I was given earlier in the thread.
It's a curve fit of liquid flow data and nominal frit thicknesses. The ROBU(Tm) charts do have one point that I find suspect; eg: they show water flow
rates are about 10x higher than air flow rates for the same pressure. That doesn't seem realistic. But I don't have another source of data to
compare against; so I'm using them anyway unless you have better data.
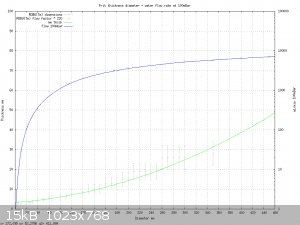
The gnuplot script to generate the plot is appended to the end of the post. It's just a text file. The script can be loaded interactively from
gnuplot, ' load "frit.gplt" ', and then the curve fit formulas become available for interactive use. The more important formula is the flow rate
calculator for water vs. pressure.
eg, you can interactively type:
print flowW( 16. , 3. , 50. ) - flowW( 8. , 3. , 50.) , flowW( 19.2, 6.8, 50 )
29.5 29.6
And gnuplot will print the flow rates for a 16mm frit, 3mm thick, with 8 mm removed from the center and a 19.2mm diameter frit, 6.8mm thick, also
gives a flow rate of 29.6 at 50 millibars. Both of them have approximately a 29 mL/minute flow rate at 50 millibars each. (100 millibars total, which
is a typical fishtank airpump pressure. )
If you want to know an approximate frit thickness compatible with ROBU(Tm) discs, just type "print thick( foo_diamter_in_mm )" and gnuplot will give
one that's close.
I drew out a complete adapter, but not the diameters of the frits; because those might change depending on what you have on hand to manufacture an
adapter with.
I calculated their diameters and thickness from a typical adapter design.
The lower frit is a 16mm washer shape with 8mm hole, 3mm thick. The upper frit is 19.2mm disk, 6.8mm thick.
If you need to make different nominal diameter frits, or drain tube, I can recompute the frit thicknesses needed to get 1/2mL per second flow rate
(30mL/minute). Let me know; or you can do it by guessing with the gnuplot script I attached.
The Gnuplot script to generate the plot, and to allow calculation is below:
Attachment: frit.gplt (2kB)
This file has been downloaded 645 times
|
|
|
officescape
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
One trick which makes these glass/ceramic melting projects easier is to use an oxy-hydrogen flame as well as feldspar as a flux.
With these two things you can do a crazy amount of exotic welding and glasswork.
I think you could grind up some glass and use a tiny oxy-hydrogen torch (electrolysis based units, either ebay or homemade) to weld it while its
sitting into a small mold.
You could then put it into position, heat the outside of the takeoff and poke dimples to hold it in place.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by officescape  | One trick which makes these glass/ceramic melting projects easier is to use an oxy-hydrogen flame as well as feldspar as a flux.
With these two things you can do a crazy amount of exotic welding and glasswork.
I think you could grind up some glass and use a tiny oxy-hydrogen torch (electrolysis based units, either ebay or homemade) to weld it while its
sitting into a small mold.
You could then put it into position, heat the outside of the takeoff and poke dimples to hold it in place. |
I imagine that's true for welding.
s there a specific advantage to oxy-hydrogen with glass welding?
I imagine a sodium feldspar would make a pretty good flux. My only concern is how repeatable a glass I could make with it if making glass from
scratch. Each time I buy feldspar, I might get a different stoichiometry.
For simple glass-making in molds; I think an electric kiln using a silicon carbide furnace ignitor for $20, might be easier for me (Im an electrical
engineer). Silicon carbide can get crazy hot -- not as hot as oxy-hrdyogen, but it's practical for kiln work.
My brother wants to make an oxy-hydrogen torch. But, I've actually pretty happy with oxy-mapp gas inside a four fire brick oven for simple jobs. It
makes a yellow-white heat easily over 1100C. That's plenty to melt borosilicate, although I'm not sure if it will fuse the precursor materials.
I've worked out a formula that I think will make Borosilicate 3.3 glass from hardware store and pharmacy chemicals.
I'm shooting for 81.1% SiO2, 12.5% B2O3, 4.2% Na2O, and 2.2% Al2O3.
Having too much sodium is the biggest problem when choosing precursor materials.
So, this is my first experimental plan:
I'm going to weigh out .0135 moles of oxalic acid ~= 17.019 grams of hardware oxalic acid (dihydrate).
Mix that with diluted waterglass, which will strip off the sodium and precipitate a very fine silica gel as a dust; so I don't have to grind up glass
-- and it's chemically pure once washed with only a trace amounts of sodium.
Then I'll mixed the washed silic acid preciptate with:
0.337 grams of hydrated alumina; ( 0.004315 moles Al[OH]3) (can be made with ammonia and aluminum foil).
2.775 grams of 20 Mule team borax. ( sodium borate decahydrate )
0.421 grams of boric acid (to keep the soda content of borax low.)
If I did the math correctly, this will provide the correct molar ratios of Na, Si, B, Al to make borosilicate glass. Hopefully the oxides and
hydroxides will change, appropriately, during fusing.
Most of the contents are water soluble, and they can easily be homogenized in a erlenmeyer flask with stir rod. That makes a pretty straight forward
mix that should oxidze and fuse into borosilicate glass; and most importantly, it should be repeatable.
Once I try it, I'll post pics and run some tests to see if the formula needs adjusting.
[Edited on 3-5-2018 by semiconductive]
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Progress report on buying custom glassware:
Deschem has gone silent .... Chemtex hasn't bid ... so I'm not sure custom glassware is even possible. Here's a copy of my conversation with DESCHEM
on ebay.
So, it looks like I'm still going to have to make it myself. 
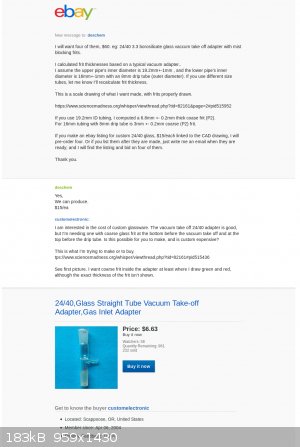 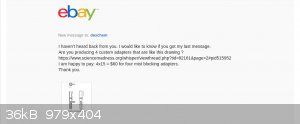
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Progress report on making borosilicate: Partial success in making ultra-fine silica precursor.
I attempted to make a known number of moles of silic acid jelly that would dehydrate to microfine silica particles.
I thought I could do it by titrating sodium metasilicate with a known number of moles of oxalic acid to neutral Ph. That avoids dehydrating the jelly
to weigh it, which converts a fair amount of it to silicon dioxide again (undesirable).

For my first attempt, I tried to make 0.1350 moles of silicic acid (eg: enough to make 10g of borosilicate glass) There is a typo in the formula for
borosilicate glass I gave two posts back ... The formula shhould say 0.1350 moles, not .01350moles.
Oxalic acid (dihydrate) is 126.064 g/mol, :. 0.1350 is 17.019 grams of oxalic acid.
I weighed oxalic acid from Ace hardware (assumed dihydrate) added to 700ML RI H2O.
I bought Humco, Houshold Farm (TM), brand "sodium silicate" to titrate with.
I added the sodium silicate to boliing and rapidly stirred 700mL oxalic acid solution. In theory, nothing should happen until the solution gets
near neutral Ph, at which point the fluid should rapidly produce a silica jelly that is very weak.
I used litmus paper to monitor the pH of the solution, and when that showed pH 7 ... I stopped adding sodium silicate. The solution boiled all-night
with vigorous magnetic stirring. Unfortunately all the water evaporated except 200mL of water or so. (A mistake.)
I then attempted to vacuum filter the jelly that was left. It was very slow, so I tried adding vinegar (5%) to make the sodium oxalate more dilute
and soluble. That worked, and I was able to vacuum filter the jelly which was very soft, smooth and of a pasty consistency.
I then rinsed the jelly with methanol, to drive out as much and oxalic acid salt as possible.
Unfortunately, something is wrong with the theory of the experiment. When I tried heating the sodium oxalate solution to dryness to drive off
methanol and acetic acid -- in theory, 0.1350 moles of di-sodium oxalate should have been left in the flask. (18.09g @ 134g/mol (wikipedia));
I did not get anywhere near 18 grams of residue. A small portion of the sodium oxalate browned (1.9g) at 125C -- so probably a contaminant.. The
rest appeared to be soldium oxalate because it did not dissolve in pure methanol.
But, the total salt residue was only 11grams. I'm not sure what's wrong.
I checked the literature on manufacture of sodium silicate ... I found sodium-metasilicate is seldom precision made in a fixed sodium:silicon
stoichiometry. Commercial preps can contain either excess or deficit of sodium hydoxide.
I took silica sand from the beach, and did a test on making sodium silicate using Lye. It is in fact easy to make, but the stoichiometry of silica to
sodium does vary depending on how I cooked the material. eg: more or less sand will dissolve in the same amount of lye.
So, I'm still not sure on how to make a specific amount of pure silica gel.
However!
I heat dried a small sample of the jelly, and it reduced to a fine silica glass dust as expected. I would estimate 300 grit or finer dust was made.
So, I'm quite happy with the quality of the silica glass made by this method. I just don't know how to make fixed quantities without lots of
sampling, dehydrating, and measuring of every silicate I buy.
Note: According to several patents, ammonia silicate sol should be impossible to produce from silica gel. But I needed to wash my vacuum filter, so
I tried ammonia anyway -- and to my delight, the jelly dissolves in 10% Ace hardware ammonia.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Update on making glass frit and still: End of May 2018.
1) Idea of hardening Vacuum Pump against chemical attack: It's finished, works great for vacuum filtration. Will show photos in next post.
2) Idea of using Glass Floss: The glass floss is here. It will take a few tries before I get it to fit the way I want.
3) Idea of making glass frit, and melting it:
The stainless steel 40 mesh screen has come, and I made a sieve out of it. Total cost ( $3 ).
I was going to use hardware store window screen for 20 mesh, and use that to sift smashed borosilicate glass ; but the harware store stuff was more
expensive ($25). And the "patch" size was too small to make a sieve. So I decided to go ahead and order 20 mesh in stainless. Expect another two
week delay.
4) Idea of buying custom glassware:
Deschem still hasn't replied to me on ebay ... so I'm beginning to doubt Deschem actually understands English well enough to be able to make custom
glassware. I've tried writing other sellers in chineese using google translate; I used google translate, even checking the translation by
translating it back into English ... so I know the translation is consistent; always gets chineese sellers to react negatively. So, either the
translation is bad ... or it sounds "dumb" to them.
Does anyone out there know chineese, natively? Care to help?
I'm not going to write Deschem in Chineese myself; I'm pretty sure that avenue of glass making is dead without a Chineese American involved as
translator.
5) Idea of making borosilicate glass from scratch:
Still workable, but requires lots of experimentation.
I discovered that although freshly precipitated silicic acid from waterglass does dissolve easily in ammonia water ... it will NOT dissolve in amonnia
if allowed to dry out in air before redissolving in ammonia. I think this is the probable reason that many sites report that silicic acid can't be
dissolved by weaker alakalines than sodium or potassium.... eg: Why cation exchange is supposedly "required" to make an ammonium sol. Once dried,
the gel will not re-dissolve in ammonia.
Therefore: Caution, anyone trying to repeat my experiments Allowing waterglass neutralized by acid to dry in a fritted buchner funnel will ruin the
funnel.
Nothing short of lye will get it out of the frit, once dried, and that will etch the frit.
Therefore, I bought a 1L porous plate vacuum buchner funnel. I can treat filter paper with silicone (diluted with mek) to make a durable filter that
can be thrown away if I ruin it. ( Pictures, later. )
So, at this point ... the biggest problem in making borosilicate glass is getting the stoichometry of silica correct using water glass.
Anyone have suggestions? Right now, I think the best that can be done is precipitate the silica, dry it, weigh it ... then redissolve it in lye; That
will give me an exact measurement of silica content in the liquid.
Then I can re-precipitate the sodium silicate; wash it to get rid of sodium... and then re-dissolve the wet silica gel in ammonia.
The process seems like a pain; but I can't think of another way to get a known amount of silica into a liquid solution without sodium present.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Aquarium pump chemical hardening (proof of concept):
Here's a photo essay about converting an aquarium pump to be both a mild vacuum pump and recirculator. It can be used for vacuum drying things,
distilling things, etc. The pump works about as effectively on a buchner funnel as a water tap venturi. Aquarium pumps don't generate strong
vacuum, but it's convenient. On top of not wasting water, I'm able to turn it off and on using a computer.
My first idea was simply to coat a pump with a mix and oil or MEK (methyl ethyl ketone) with silicone. Letting the pump suck up and spit out the mix
will protect any metal or rubber parts inside the pump against harsher chemical fumes. Silicone won't withstand pure sulfuric acid, but it can handle
even 90% intermittently. This method does work, as long as the silicone is very thin; but NOT if there is a check valve installed. I originally
drilled a hole on top of the pump where the check valve was, but then changed my mind and drilled out the side of the pump and bypassed the check
valve.
Aliaphatic oils, like lighter fluid or silicone waterproofing fluid (purchased in an aerosol can from the store) will thin silicone just as
effectively as MEK and not hurt a check valve; However, the evaporation rate is very slow. This results in the silicone hardening before the oil is
released. This is undesirable because it means that the oil is reducing the silicone's integrity. So, I used MEK and got rid of the check valve.
The pump is a whisper 10, 10 gallong aquarium pump.
All aquarium pumps use 3/16 inch ID tubing. Typically, Vinyl.
Pure GE silicone caulk or fishtank silicone is acetoxy cure. Acetoxy silicones are formulated to cure in thick (1/4) slabs. This means they are
designed to have 1 micron sized pores in them to allow air vapor to pass through, slowly. They cure to a hazy finish.
OTOH, cheap alkoxy cure silicones (Nanda, From China #704, #705 ) do not cure in thick layers. The clear silicone is optically clear. I believe this
means they are not porous on the scale of 200nm or more. Therefore, they ought to be nearly air tight.
I used #704 (white) so that it would show up in the photos of the pump conversion. It's also the stronger silicone. I hand painted the rubber
diaphragm, inside and out, with three thin coats of silicone. When the black rubber and metal screw was no longer visible inside the diapgram, I
quit.
I'm not sure what causes the white color, and it may reduce chemical resistance. I may attempt to fumigate the pump with fluorine gas to make a
protective coating on the surface of the silicone, eg: if I can figure out how to generate fluorine gas. Likewise, the flapper valves in the white
pump body are just a piece of flat plastic and could probably be replaced with teflon tape for better chemical resistance.
For this first attempt, I simply glued in clear vinyl tubing (fishtank air hose) with marine epoxy. I then potted the vinyl pipe with silicone
(thinned with mek) ... in several 1mm layers.
I will be replacing the vinyl leader hose with 5mm glass pipe, later. The reason is that plastic connectors I get from the fishtank supply house
can't handle MEK or strong solvents, and vinyl tubing, softened by MEK, collapses under vacuum.
I plan to change to glass connectors and use platinum cured silicone vacuum hose. Silicone hose with 3/16 ID and 3/8 OD is not expensive and can
handle vacuum even after MEK.
Enjoy the photos:
 
 

[Edited on 12-6-2018 by semiconductive]
|
|
|
| Pages:
1
2 |
|