| Pages:
1
2 |
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Jackpot from abandoned institute of inorganic chemistry
Scored some good stuff from abandoned place, had some day job business there, decided to take a look around and found some useful stuff.
Most of it is cleared out, but still. ( sadly went past empty storage room of hydrates).
No pictures of the place itself, because it was mostly empty and was not that interesting.

Sorting it all out right now, I'll update what did I got!
|
|
|
MJ101
Hazard to Self
 
Posts: 82
Registered: 14-6-2018
Member Is Offline
Mood: Always Sunny
|
|
That looks like a nice find. 
Best of luck with everything there.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
This is what I got, lazy excel, sorry for that! Quantity is approximate and given in grams.
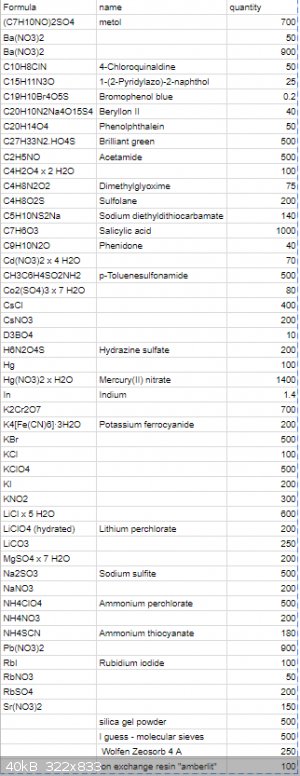
There is a mistake:
C4H2N2O4 · H2O Alloxan monohydrate 100g
As a Bonuss - I give you 2 mystery substances:

A and B (both might be related in some way)
A is a grey powder, not really soluble in water.
B is dark brown powder even less soluble in water.

A substance dissolves in HCl forming yellow solution.
B substance is not dissolving well in HCl.

A substance dissolves in H2SO4 and forms pinkish solution.
B substance dissolves in H2SO4 and gives brownish solution.
A substance colors flame greenish, confirms suspicion of boron being present.
B substance decomposes in flame giving black foam, might be organic compound. colors flame yellow, not really a hint. might be carbon.
What should I do next?
[Edited on 10-7-2018 by TheMrbunGee]
[Edited on 10-7-2018 by TheMrbunGee]
|
|
|
Vosoryx
Hazard to Others
  
Posts: 282
Registered: 18-6-2017
Location: British Columbia, Canada
Member Is Offline
Mood: Serial Apple Enjoyer
|
|
I'm amazingly jealous. What a find.
Some pretty obscure ones in there too.
"Open your mind son, before someone opens it for you." - Dr. Walter Bishop
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
350g of rubidium salts? Not that useful honestly, but that's a small fortune!
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Looks like someone may have made custom fireworks??
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
As an institute of inorganic chemistry - I think the had huge variety of different chemicals, these I got from just 2 cabinets someone forgot to
clear. I don think they were making fireworks in there. (maybe just for fun once in a while)
And I left a lot there, took only things I found interesting. (and could store at home. (there was a jar of anhydrous aluminum chloride, that I had to
leave there, because it was sitting in sulfuric desiccator and it would be hard to transport and I don't have a room for that setup))
| Quote: |
350g of rubidium salts? Not that useful honestly, but that's a small fortune!
|
yea, there are many things I would never pay for, but now that I have them - I can explore some properties.
So no one is up for the challenge?
|
|
|
MJ101
Hazard to Self
 
Posts: 82
Registered: 14-6-2018
Member Is Offline
Mood: Always Sunny
|
|
Is substance A some sort of Chromate?
I was reading about it here:
http://www.chem-toddler.com/chemical-equilibrium/chromatedic...
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Wow... A kilo and a half of mercury salt... Weren't you even a bit scared to take that thing home?
Also, substance A may very well be a barium compound as those color the flame greenish, while boric acid colors it bright green.
If I were to guess based on your results, I'd say the black powder is either MnO2 or Mn2O3 just because it gives that solution is H2SO4 (which looks
like Mn (III) from the photo) and isn't soluble in water. As for the HCl reaction, MnO2 and especially Mn2O3 sometimes reacts quite slowly with
room-temperature dilute aqueous HCl, so it might seem insoluble.
Substance A is really weird as I don't know any compound with all those properties. It might be some MnCO3 contaminated with Fe2O3 though, but that
wouldn't explain the green flame and would only account for the colors of the solutions.
I might be totally wrong here, but this is the only explanation I could find.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
And the Cs !
But I wouldnt know what to do with 1400g of Mercury salts except be scared!
I'm still jealous though 
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Excitement was overwhelming.  haven't opened any of Hg containers yet. haven't opened any of Hg containers yet.
__________________________________________________
I have a strong feeling that both of them are related to boron. (because of location where I got them)
B barely decomposes H2O2 (10%)
 , ,
It does, when I added NaOH solution, but not as intensive as it should.

Today I looked at B in daylight and it is more like dark brown-yellow. maybe a hint of green.

B powder dropped in warm concentrated NaOH solution clumps it op and makes it almost black,

After stirring it partially dissolves, (same solution more exposed on the right)
 
In 28% HCl after warming, it dissolves and forms solution in the color of baby vomit (left pic.)
Something sinks down, but its much lighter in color.
I don't have distilled water, but after I added tap water to the HCl solution yellow color was gone, and some white-ish particles formed and sank to
the bottom, next to the particles that did not dissolve in the first place(right).
 
Wouldn't carbonate release CO2 in acid?
And I don't think any of them are chromates.
[Edited on 11-7-2018 by TheMrbunGee]
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
How does A react with H2O2? I really don't know any compound that is pink in H2SO4 solution besides those of Mn(II) and those would catalyze the
decomposition. What seems odd to me is that the solution of A in H2SO4 doesn't really look like MnSO4 and looks more like ferrate (VI), but that
wouldn't exist in acid solution.
I don't think either of them contains boron because they should be inorganic considering they are from an inorganic chemistry institution, and the
only inorganic compounds of boron which are stable in water and acid solution are the borates, but those would be colorless.
[Edited on 11-7-2018 by CobaltChloride]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by CobaltChloride  |
I don't think either of them contains boron because they should be inorganic considering they are from an inorganic chemistry institution, and the
only inorganic compounds of boron which are stable in water and acid solution are the borates, but those would be colorless.
[Edited on 11-7-2018 by CobaltChloride] |
They might not, true. but the place had plenty of organic stuff, so it might be organic.
_______________________________________
A substance slowly decompose H2O2,

After I heated the mixture, decomposition was fast and self sustaining.

And I'm left with yellowish solid after H2O2 was spent.

Also I found that substance A reacts with methanol, forming greenish solution (it does not dissolve completely, but colors both - methanol and solid
dirty green color) which after an hour have turned more brown.

After shaking up.

Nothing interesting happens with both substances in:
Ethanol
Methanol (for substance B)
DCM
__________________________________________
Edit:
More on MeOH and substance A:
On combining of two, solution and solid turns green

On heating turns brown in about 30 secounds

Solution burns with green flame

[Edited on 11-7-2018 by TheMrbunGee]
|
|
|
Hunterman2244
Hazard to Others
  
Posts: 105
Registered: 6-6-2018
Member Is Offline
|
|
WishI had stuff like that around me. The substance a seems like something copper containing, maybe some complex. Was thinking organic, but its an
inorganic chem institute.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by Hunterman2244  | | WishI had stuff like that around me. The substance a seems like something copper containing, maybe some complex. Was thinking organic, but its an
inorganic chem institute. |
I have not obtained any copper salt typical color.. And as I said there was plenty of organic substances, I even have some in my list!
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
That flame definitely looks like something containing either boron or copper (as hunterman said). I would be more inclined to think it is copper as
all the organics you had there were common chemicals (nothing exotic), but boranes and borate esters are somewhat more exotic.
The results of the test you did are very weird. Both substances behave as a mix of different compounds, especially regarding color.
[Edited on 11-7-2018 by CobaltChloride]
|
|
|
Hunterman2244
Hazard to Others
  
Posts: 105
Registered: 6-6-2018
Member Is Offline
|
|
Quote: Originally posted by CobaltChloride  | That flame definitely looks like something containing either boron or copper (as hunterman said). I would be more inclined to think it is copper as
all the organics you had there were common chemicals (nothing exotic), but boranes and borate esters are somewhat more exotic.
The results of the test you did are very weird. Both substances behave as a mix of different compounds, especially regarding color.
[Edited on 11-7-2018 by CobaltChloride] |
That's what I was thinking, some organic copper complex. The brown color made me think of something organic, maybe reacting with the methanol and
releasing copper ions.
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
I wouldn't think an organic complex would be stable inside a flame, but A was stable enough for TheMrbunGee to do a flame test.
|
|
|
phlogiston
International Hazard
    
Posts: 1375
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Note that thallium imparts a similarly green color to flames. I don't know enough about Tl chemistry to tell if your other tests are compatible with
it being a compound of Tl, but you might want to be careful about handling it until you know its identity.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by phlogiston  | | Note that thallium imparts a similarly green color to flames. I don't know enough about Tl chemistry to tell if your other tests are compatible with
it being a compound of Tl, but you might want to be careful about handling it until you know its identity. |
You actually got me scared for a minute. 
___________________________________________________
Ok, did some tests.
First I combined both substances with ammonia solution.
A gained a slight blue hint. (B left; A right)

B actually dissolved. Left Pine green solution. (shining light trough B solution)

Next I decomposed both substances by heating (yes, both of them decomposes:
A leaves black clumps, releases quite a lot of water and some tar. ;
B foams up, releases some water and leaves black to dark brown solid foam . )
Products of decomposition Were added to HCl 28%:
A - turned copper sulfate blue

B - nothing.

Added some H2O2 (10%) :
A turned copper chloride green

B turned yellow,

After mixing,

After heating some hint of green was observed.

A is copper for sure.
B - Not sure yet. But maybe.
Both organic.
[Edited on 11-7-2018 by TheMrbunGee]
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
It can't be thallium. Thallium is well known to have very poorly soluble halides, but the substance which turns the flame green dissolves in HCl.
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Substance A has the appearance of copper Iodide, CuI. It's a water insoluble beige/gray solid where Cu has +1 oxidation state and can give rise to
intermediate complexes with different colorations (among which even the yellow you see with HCl). The pink in H2SO4 actually reminded me of cobalt,
but that green flame is a giveaway of copper.
In many of those reactions something turns Brown. That could be iodine forming. Did you smell any iodine? Usually the smell is very distinct. On
heating, do you get purple vapors?
Substance B is a bit unusual if it's only inorganic. It Looks like manganese oxide but the reaction products you get afterwards look more like some
iron II/III mix, while that teal green coloration you get with ammonia looks like nickel II.
If you want to carry out additional tests, I'd separate any solid from that solution and attempt some redox reaction of whatever is leftover. Also try
and see if Aqua regia destroys the black lumps.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by Metallus  | Substance A has the appearance of copper Iodide, CuI. It's a water insoluble beige/gray solid where Cu has +1 oxidation state and can give rise to
intermediate complexes with different colorations (among which even the yellow you see with HCl). The pink in H2SO4 actually reminded me of cobalt,
but that green flame is a giveaway of copper.
In many of those reactions something turns Brown. That could be iodine forming. Did you smell any iodine? Usually the smell is very distinct. On
heating, do you get purple vapors?
Substance B is a bit unusual if it's only inorganic. It Looks like manganese oxide but the reaction products you get afterwards look more like some
iron II/III mix, while that teal green coloration you get with ammonia looks like nickel II.
If you want to carry out additional tests, I'd separate any solid from that solution and attempt some redox reaction of whatever is leftover. Also try
and see if Aqua regia destroys the black lumps. |
I did not detect any iodine. I know the smell and it was not there. decomposition of A substance gave a nasty stench, but nothing distinct I could
recognize. some tar formed - it could be anything.
Substance B is also organic. Black lumps seems to be carbon. in both cases.
I'll try something, but not now, I'll update!
|
|
|
Schleimsäure
Hazard to Others
  
Posts: 156
Registered: 31-8-2014
Location: good ole Germany
Member Is Offline
Mood: Probably
|
|
Good find. In Germany I suppose?
Went on the hunt here too 2 or 3 years ago. But was not very lucky.
Indeed I was very unlucky.
Here I came appr. 1 year too late:

In another similar one I came just a few months too late and got only a handful of nice empty Merck bottles and an empty 5l benzene flask (former
GDR).
Former Eastern Germany has still some "treasures", there was so much chemical industry in the former GDR, which only got abandoned at some point after
1990.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Looks amazing!
No I am in Latvia, and this institute seems to be abandoned recently, I found some notes from 2004. but they might as well be left there after shut
down.
and I saw pictures from 2016 when it was full of jars stacked in piles to be utilized. I am really sad, I did not know about that place earlier..
|
|
|
| Pages:
1
2 |