MattEx
Harmless

Posts: 12
Registered: 30-5-2005
Location: Auckland, New Zealand
Member Is Offline
Mood: No Mood
|
|
2,4,6-cycloheptatriene-7-carboxylic acid
Anyone know a procedure for adding a carboxylic acid group to the right position of 1,3,5-cycloheptatriene to yield the named target product  ? ?
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Is that named correctly? I would have thought 2,4,6-cycloheptatriene carboxylic acid or 2,4,6-cycloheptatriene-1-carboxylic acid would be better
names. However, I am no IUPAC expert by any means. Now you have an addition or substitution reaction to a conjugated cyclic alkene. Can you Google
some information about the next step?
Here's a good starting place:
http://www.cem.msu.edu/~reusch/VirtualText/intro1.htm
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
2,4,6-cycloheptatriene fairly readily loses an hydride ion, e.g. by reaction with a sufficiently stable carbonium cation like the triphenylmethyl
cation, to become the resonance-stabilized planar tropylium cation, C7H7(+). So any reaction procedure involving an intermediate in the form of a
carbonium cation, in this hypothetical case (+)COOH, is liable to have the same effect
|
|
|
Leopata
Harmless

Posts: 4
Registered: 6-10-2011
Member Is Offline
Mood: No Mood
|
|
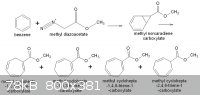
Benzene + methyl diazoacetate = methyl cycloheptatriene carboxylic acid methyl ester, via norcaradiene methyl ester.
The corresponding norcaradiene carboxylic acid, at high temperatures rearranges with ring expansion to the cycloheptatriene carboxylic acid methyl
ester, an important reagent to 2-tropene-2-carboxylate ring. I don´t have the literature at hand now, but with this you can start your investigation.
Hope you have what you were looking for.
|
|
|
Leopata
Harmless

Posts: 4
Registered: 6-10-2011
Member Is Offline
Mood: No Mood
|
|
Here´s the reaction scheme
|
|
|
Leopata
Harmless

Posts: 4
Registered: 6-10-2011
Member Is Offline
Mood: No Mood
|
|
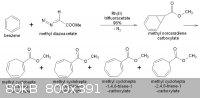
Sorry...there´s a mistake in the figure above.
[Edited on 8-10-2011 by Leopata]
|
|
|
Retard-3000
Hazard to Self
 
Posts: 55
Registered: 17-11-2010
Member Is Offline
Mood: No Mood
|
|
What do you want this 2,4,6-cycloheptatriene-7-carboxylic acid for ?
|
|
|