| Pages:
1
..
25
26
27
28
29
..
43 |
Luton
Harmless

Posts: 3
Registered: 13-11-2010
Member Is Offline
Mood: No Mood
|
|
Well I guess by using Acetyl Chloride + Sodium Acetate = Ac2O is the most common.
But what I did not understand was,why use H2SO4, wouldn't the Acetyl Chloride and Sodium Acetate react without H2SO4.
If H2SO4 is still needed, does anyone know how much to use in Lab.
Too much Googling is bad for health 
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | But what I did not understand was,why use H2SO4, wouldn't the Acetyl Chloride and Sodium Acetate react without H2SO4. |
Beats me too. . .
Anyway, keeping moisture out of the reaction is the important consideration!
http://www.erowid.org/archive/rhodium/chemistry/anhydrides.h...
|
|
|
SHADYCHASE54
Hazard to Others
  
Posts: 150
Registered: 16-12-2010
Location: CaNaDay!
Member Is Offline
Mood: No Mood
|
|
I am sorry if it seems like I'm beating a dead cat, resurrecting an old and well covered issue however, I have a question about Ac20 prep. via P2O5
dehydration. I have attempted this synthesis myself with much of the same result as previous posters, black sludge that gave nothing but my glacial
back to me. A long time ago I remember reading a paper that for the life of me i cannot place that claimed that the P2O5 dehydration works when 2
carboxylic acids are mixed producing the anhydride of each component as well as a mixed anhydride.
any thoughts...
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Yes, acetic anhydride cannot be prepared by dehydration of acetic acid . . .
|
|
|
SHADYCHASE54
Hazard to Others
  
Posts: 150
Registered: 16-12-2010
Location: CaNaDay!
Member Is Offline
Mood: No Mood
|
|
Yesm I too am aware that simple dehydration of glacial will result in glacial however my question was about a mixture of the two. Now I realize that
there might seem to be little difference between the dehydration of one as apposed to two. But like I said I believe I did at one time read a paper on
this that suggested this works. In the article the two carboxylic acids were glacial acetic & propinoic acid which were dehydrated with P.
pentoxide resulting in...
acetic anhydride + propinoic anhydride + acetic-propinoic anhydride. I have budgeted for some propinoic acid next month so come mid month I will try
to duplicate what i remember and post my results, or my lack there of.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | . . . were dehydrated with P. pentoxide resulting in...
acetic anhydride + propinoic anhydride + acetic-propinoic anhydride. |
I think that should be a chloride of phosphorus, not the pentoxide . . .
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
I had a crazy idea, maybe someone can tell me if its feasible or not.
Vinyl acetate can be reacted with acetic acid to make acetic anhydride and acetalydehyde, which escapes and drives the reaction forward. There is only
a few mentions of this reactions on this forum but it seems to be possible.
Instead of vinyl acetate my theory is isopropenyl acetate, also known as acetone-enol acetate, can be used. Heating the mixture will distill off
acetone and form the Ac2O. It also might be possible to make this at home with acetone, acetic acid, an acid catalyst and dehydration to form the enol
and create an ester.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I think you're more likely to just get a mess of aldol condensation products. 
I weep at the sight of flaming acetic anhydride.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by mr.crow  | I had a crazy idea, maybe someone can tell me if its feasible or not.
Vinyl acetate can be reacted with acetic acid to make acetic anhydride and acetalydehyde, which escapes and drives the reaction forward. There is only
a few mentions of this reactions on this forum but it seems to be possible.
|
I did this reaction many time and i am sure this method is wrong.
No anhydrid produce just acetaldehyde and tar.
Vinyl acetate wont decompose to acetic anhydrid(I promise you)
[Edited on 12-2-2011 by Waffles SS]
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Thanks. My idea was based on a patent from the 30s and we all know how accurate those can be.
I could try to buy the vinyl acetate but what fun is that?
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Vinyl acetate method(above patent) and pyrosulfate method(sodium pyrosulfate+AcONa) is scam!!(I swear)
Dont waste your time by this method.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
| Quote: |
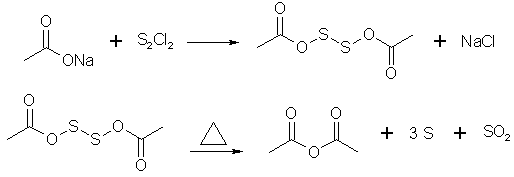
Prepare 100g freshly fused NaOAc and 65g S2Cl2. A small quantity of NaOAc is placed in a thin-walled glass cooled in an ice bath. To this is added
some sulfur chloride, the mixtr is vigorously stirred w/a wooden spatula, not allowing the temp to rise. Then some NaOAc is added again, and the
process is repeated several times until all is mixed in. The semi-liquid mass is transferred into a 1 liter RBF. The previous operation is repeated 4
times, so that 400g NaOAc and 260 g S2Cl2 total are taken into work. The RBF is then equipped w/a reflux condenser and gently heated on a water bath
to ~80-85C. As soon as the rxn starts, the heating is removed, and in case the rxn gets too vigorous it's cooled w/cold water. After 20-30 min SO2
evolution ceases and the mixture is heated for 10 more min's on a boiling water bath. The rxn product is then distilled off under vacuum, then
fractionally re-distilled at ordinary pressure, collecting the fraction boiling between 132-142C.
|
According to Stoichiometry of this reaction every 2 mol of sodium acetate need one mol of S2Cl2 this mean 400 gram sodium acetate need 329 gram S2Cl2
but this patent use 400 gram sodium acetate and 260 gram S2Cl2 !
2AcONa + S2Cl2 = AcO-S-S-OAc + 2NaCl
Also i think in this method for better mixing and better distill we need solvent (high boiling point solvent is better but which one?).
[Edited on 4-3-2011 by Waffles SS]
|
|
|
goahead
Harmless

Posts: 1
Registered: 3-1-2011
Member Is Offline
Mood: No Mood
|
|
Partial success with acetic anhydride synthesis and a question
Hello everyone,
I've tried the reaction with sodium acetate/Br2/S twice. The first reaction resulted in roughly 5ml of a clear yellowish liquid, which distilled at
55°C at a vacuum of unknown strength, with a stingy smell, which turned to acetic quickly. The liquid sank in water and dissolved in it after about
30 minutes. It burned with a weak blue flame.
Since the small amount of product wasn't sufficient for a second distillation at normal pressure, the reaction was performed one more time. This time
the sodium acetate was added to the sulfur bromide instead of the other way round, which proved to be more difficult because of bromine vapors
escaping. From 21.5g bromine 15g of a clear, pungent liquid was obtained.
This liquid also sank in water but dissolved in it rapidly. The smell was more pungent-garlic like and the acetic component almost missing / much
weaker. The liquid distilled at normal pressure in the range of 80-100°C.
Any ideas what this product might be?
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
US2073686
| Quote: |
Proocess for manufacture of acetic anhydrid, which comprises heating to a temperature between 200-450c a mixture of cupric acetate with copper salt of
a strong of hydrochloric acid and sulfuric acid
|
http://www.sciencemadness.org/talk/files.php?pid=216018&...
[Edited on 16-7-2011 by Waffles SS]
|
|
|
Bitburger
Harmless

Posts: 41
Registered: 23-7-2011
Location: Belgium
Member Is Offline
Mood: Experimental
|
|
With 2 simple chemicals: sodium acetate and ammonium chloride!
When you mix it up and do a dry distillation, then a aqueous solution of ammonia comes over first, with some acetamide in it. But if all water is
boiled, temperature rises and the destillation apparatus is then filled with smoke: a oily liquid floating on the top will come over. This is ethanoyl
anhydride.
I prepare it by incident - if you read my topic "Synthesis of propanamide" then you will see the yellow propanoyl anhydride.
By heating it up with NaOH, the mixture became a solid, waxy compound when cooled. This is sodium propanoate. Furthermore, it is very soluble in
diethyl ether - like propanoyl anhydride. Also, the density was established.
I still have a sample of it.
[Edited on 30-7-2011 by Bitburger]
Good to be wrong
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Did you test your Acetic anhydrid?really that is acetic anhydrid or acetic acid?
I confirm that dry distillation of sodium acetate and ammonium chloride produce acetamide but i Doubt acetic anhydrid may produce by this method
|
|
|
Bitburger
Harmless

Posts: 41
Registered: 23-7-2011
Location: Belgium
Member Is Offline
Mood: Experimental
|
|
Quote: Originally posted by Waffles SS  | Did you test your Acetic anhydrid?really that is acetic anhydrid or acetic acid?
I confirm that dry distillation of sodium acetate and ammonium chloride produce acetamide but i Doubt acetic anhydrid may produce by this method
|
No, I confirmed that propanoyl anhydride was formed if you are patient. Common practice said to me that this method also must work for making acetic
anhydride. Maybe it don't work for the synthesis of ethanoyl anhydride...
Did you ever performed this reaction with sodium ethanoate and ammonium chloride until the distillation apparatus was filled with smoke?
Good to be wrong
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bitburger  | With 2 simple chemicals: sodium acetate and ammonium chloride!
When you mix it up and do a dry distillation, then a aqueous solution of ammonia comes over first, with some acetamide in it. But if all water is
boiled, temperature rises and the destillation apparatus is then filled with smoke: a oily liquid floating on the top will come over. This is ethanoyl
anhydride. |
Signature:
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Common practice said to me that this method also must work for making acetic anhydride. |
Common sense would suggest that if it were that easy, we would not have a thirty page thread devoted to it. Or do you think you are the first person
to combine these chemicals and heat them? They were known to the alchemists even. Anyway, the anhydrides of carboxylic acids get progressively harder
to make as the number of carbons decreases; many methods that might be applicable to the synthesis of propionic anhydride would not work for acetic
anhydride. Which is not to say that I think you made propionic anhydride either.
|
|
|
Bitburger
Harmless

Posts: 41
Registered: 23-7-2011
Location: Belgium
Member Is Offline
Mood: Experimental
|
|
A hypothetical consideration is the reaction between acetic acid and acetaldehyde in the presence of a base. The last step could be achieved by a mild
oxidation.
Click on the picture to enlarge
Good to be wrong
|
|
|
simba
Hazard to Others
  
Posts: 175
Registered: 20-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bitburger  | A hypothetical consideration is the reaction between acetic acid and acetaldehyde in the presence of a base. The last step could be achieved by a mild
oxidation.
Click on the picture to enlarge |
Looks too good to be true...I think it is more likely to get just acetate salts and water from this.
|
|
|
MeSynth
Hazard to Others
  
Posts: 107
Registered: 29-7-2011
Member Is Offline
Mood: http://www.youtube.com/watch?v=5ZltqlVuDIo
|
|
When all else fails.... http://www.elementalscientific.net
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I stumbled into a neat way to make acetyl chloride. I make no claims that it is easier than the alternative routes, but I thought I'd bring it up
since no one has.
Alkyl disulfides in glacial acetic acid react to form alkyl sulfenyl chlorides and acetyl chloride. The AcCl can be distilled out of the mixture. The
sulfenyl chloride could be recycled back into disulfide, or used to make other things such as sulfinyl chloride, sulfinic acid, sulfonyl chloride, or
sulfonic acid.
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV5...
The required alkyl disulfide can be easily made from an alcohol via the alkyl bromide reacting with either sodium polysulfide or thiosulfate. Pretty
easy looking stuff.
|
|
|
Bitburger
Harmless

Posts: 41
Registered: 23-7-2011
Location: Belgium
Member Is Offline
Mood: Experimental
|
|
Quote: Originally posted by shivas  | Quote: Originally posted by Bitburger  | A hypothetical consideration is the reaction between acetic acid and acetaldehyde in the presence of a base. The last step could be achieved by a mild
oxidation.
|
Looks too good to be true...I think it is more likely to get just acetate salts and water from this. |
What happens with this reaction when a non-nucleophilic base, e.a. Et3N is used
instead of a hydroxide salt?
Good to be wrong
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Manufacture of fatty acid anhydrides - Henry Dreyfus - US patent number
2073686
Almost a year ago, after receiving a power supply in the post, I decided to give it a test making some copper acetate using acetic and copper wire. I
then distilled the results, mainly out of curiosity to see roughly how it behaved rather than achieve anything significant.
Fast forward and I was chatting with someone from the forum who mentioned seeing a patent, the patent above, regarding the production of anhydrides
from acetates. The patent specifically mentions copper acetate being distilled with copper sulphate. Both the salts are dried prior to distillation.
Approximately 30 to 60% w/w of the sulphate is added per unit of acetate.
Some might call this squirreling, but...
I still had the finely powdered copper left over from the last time I made copper acetate:
<img src="http://img85.imageshack.us/img85/3724/img1939jk.jpg" width="400" />
I decided to try the distillation again, but this time including copper sulphate and well drying the materials prior to use.
I combined the piles of copper powder in a small beaker, checked the mass and then added Glacial Acetic. On top of that, I dripped in some 35%
peroxide. The reaction is somewhat busy. I then left it to bubble away on a hotplate for about thirty minutes to get it all (hopefully) to the (II)
acetate.
Meanwhile, I found a lump of copper sulphate I had been watching turn into crystals for the last year and scooped them out.
The acetate by now had absorbed so much of the acid it I could turn the beaker upside down and it wouldn't drip. I emptied that onto a large watch
glass and the sulphate into a petri dish and put both in the oven at around 75C for six hours. Periodically removing them to crush them (particularly
the sulphate) into dust.
The glassware was left in the oven for a few hours to ensure that was also dry.
I then put the acetate under about 80mbar of vacuum and heated that to 140C for four hours, leaving the bottle capped after removing it. I repeated
the process for the sulphate but allowed it to become a colourless mass (which should be anhydrous, or close).
I emptied all of the acetate into a preweighed flask and determined that I had about 16g of it. Then was added 60% of this w/w of the sulphate. The
two were shaken together and set up for simple vacuum distillation.
The powders are notably free flowing, to the point that they are carried through a still by the vapours. It occurs readily enough to line the entire
still, as per the photos in the other thread. This time, I wadded the base of the column with some glass wool. Despite this, I could still see faint
traces of powders filtering through the wool. It was, however, no where near as bad as the example in the other thread.
The distillation was run around 80mbar and I was recovering a colourless liquid at 35C into a preweighed flask. When the temperature fell, I checked
the flask and it was now entirely brown, indicating the end. I had recovered approximately 5g of liquid. This was placed in the freezer at -17 / -
18C. Returning shortly after, it had all frozen solid. A bad sign in terms of acetic anhydride, as it's melting point is -73.1C, so there must be at
least some moisture in there.
I then swapped it over to the fridge. I had two digital thermometers in there reading between 7 and 8.6C. I have also had some flasks of cyclohexane
in there for months, which are constantly going into and out of solids and liquids, or half and half. The melting point of cyclohexane is 6.5 to 7C,
so that seems realistic. The material in the flask also became a liquid, over the coarse of many hours. It's own MP will be somewhere close to that of
cyclohexane.
Inspecting the flask I could see traces of the powdered reagents in there. It was a faint baby blue, indicating copper sulphate monohydrate.
I then decided to distill it through a column, but at atmospheric, such that I could check that BP. Which I did, with the aid of some argon and CaCl2
on the outlet. The BP of the anhydride is 139.8C, that of GAA is 118 to 119C. The Y is in Celsius, the X is time, in minutes.
<img src="http://img851.imageshack.us/img851/4886/screenshotat20111226210.png" width="400" />
Not looking too good for the anhydride!
To be more sure, I titrated 1ml of the liquid and obtained an acetic molarity of 17.9, with the molarity of GAA being 17.5M.
Comments
YAY! I've made acetic acid!
Errors and improvements
The hydration states of the materials are obviously very important if the goal is the anhydride. In the patent, Henry specifically mentions that the
acetate may be not entirely anhydrous, but the sulphate should be as close as possible.
The acetate is difficult to dry without accidentally decomposing. Hard vacuum would be a good idea.
I don't think checking the hydration states by the signs of decomposition or colourloss alone are going to be good enough. It seems to require that
they are insured with mass checks.
I started with a known mass of copper, but in an unknown oxidation state. So I could estimate the amount of acetic that would be required, but I could
not actually check which acetate I had and if I had solely that acetate. I relied instead on it simply being that acetate due to the excess of GAA and
heating.
On that point, the amount of GAA yielded seems low by comparison with the mass of liquid (likely GAA, or something close) that I have received back.
Indicating that the mass of acetate I weighed after drying was either partially decomposed (doubt that's significant, it was all green), partially the
lower (I) acetate (it was definitely green), still damp to some degree (decomposition had just begun as I turned off the heating, indicating it must
be close to fully dehydrated) or a combination of all three.
I spent about a day in total drying glass and salts for this, and have yielded bum all anhydride, indicating the importance of keeping track of the
actual mass changes occurring. If I try this again, I will be following it from start to distillation with carefully checked masses and states. I may
also use an excess of the sulphate.
I could do with getting some aniline, to follow Len's suggestion to ?Lambda? of using that to check his results.
Obligatory photos
<a href="http://img689.imageshack.us/i/img1940b.jpg/" target="_blank"><img src="http://img689.imageshack.us/img689/8909/img1940b.jpg"
width="400" border="0"/></a>
<a href="http://img651.imageshack.us/i/img1944p.jpg/" target="_blank"><img src="http://img651.imageshack.us/img651/4321/img1944p.jpg"
width="400" border="0"/></a><br>
<a href="http://img853.imageshack.us/i/img1945z.jpg/" target="_blank"><img src="http://img853.imageshack.us/img853/3432/img1945z.jpg"
width="400" border="0"/></a><br>
<a href="http://img850.imageshack.us/i/img1946pq.jpg/" target="_blank"><img src="http://img850.imageshack.us/img850/3882/img1946pq.jpg"
width="400" border="0"/></a><br>
<a href="http://img69.imageshack.us/i/img1947q.jpg/" target="_blank"><img src="http://img69.imageshack.us/img69/9256/img1947q.jpg"
width="400" border="0"/></a><br>
<a href="http://img685.imageshack.us/i/img1948nj.jpg/" target="_blank"><img src="http://img685.imageshack.us/img685/2521/img1948nj.jpg"
width="400" border="0"/></a><br>
<a href="http://img607.imageshack.us/i/img1949aj.jpg/" target="_blank"><img src="http://img607.imageshack.us/img607/7306/img1949aj.jpg"
width="400" border="0"/></a><br>
<a href="http://img828.imageshack.us/i/img1951p.jpg/" target="_blank"><img src="http://img828.imageshack.us/img828/6963/img1951p.jpg"
width="400" border="0"/></a><br>
<a href="http://img802.imageshack.us/i/img1952d.jpg/" target="_blank"><img src="http://img802.imageshack.us/img802/4994/img1952d.jpg"
width="400" border="0"/></a><br>
<a href="http://img546.imageshack.us/i/img1955a.jpg/" target="_blank"><img src="http://img546.imageshack.us/img546/3637/img1955a.jpg"
width="400" border="0"/></a><br>
<a href="http://img40.imageshack.us/i/img1956r.jpg/" target="_blank"><img src="http://img40.imageshack.us/img40/8069/img1956r.jpg"
width="400" border="0"/></a><br>
<a href="http://img688.imageshack.us/i/img1957r.jpg/" target="_blank"><img src="http://img688.imageshack.us/img688/6863/img1957r.jpg"
width="400" border="0"/></a><br>
<a href="http://img14.imageshack.us/i/img1958b.jpg/" target="_blank"><img src="http://img14.imageshack.us/img14/6439/img1958b.jpg"
width="400" border="0"/></a><br>
<a href="http://img266.imageshack.us/i/img1959br.jpg/" target="_blank"><img src="http://img266.imageshack.us/img266/5479/img1959br.jpg"
width="400" border="0"/></a><br>
<a href="http://img846.imageshack.us/i/img1961hy.jpg/" target="_blank"><img src="http://img846.imageshack.us/img846/2680/img1961hy.jpg"
width="400" border="0"/></a><br>
<a href="http://img26.imageshack.us/i/img1964vc.jpg/" target="_blank"><img src="http://img26.imageshack.us/img26/7894/img1964vc.jpg"
width="400" border="0"/></a><br>
<a href="http://img522.imageshack.us/i/img1968km.jpg/" target="_blank"><img src="http://img522.imageshack.us/img522/6482/img1968km.jpg"
width="400" border="0"/></a><br>
<a href="http://img849.imageshack.us/i/img1971p.jpg/" target="_blank"><img src="http://img849.imageshack.us/img849/3220/img1971p.jpg"
width="400" border="0"/></a><br>
<a href="http://img850.imageshack.us/i/img1974gt.jpg/" target="_blank"><img src="http://img850.imageshack.us/img850/3257/img1974gt.jpg"
width="400" border="0"/></a><br>
<a href="http://img515.imageshack.us/i/img1976f.jpg/" target="_blank"><img src="http://img515.imageshack.us/img515/3905/img1976f.jpg"
width="400" border="0"/></a><br>
<a href="http://img97.imageshack.us/i/img1978k.jpg/" target="_blank"><img src="http://img97.imageshack.us/img97/809/img1978k.jpg" width="400"
border="0"/></a><br>
<a href="http://img41.imageshack.us/i/img1979wk.jpg/" target="_blank"><img src="http://img41.imageshack.us/img41/8149/img1979wk.jpg"
width="400" border="0"/></a><br>
<a href="http://img542.imageshack.us/i/img1982q.jpg/" target="_blank"><img src="http://img542.imageshack.us/img542/6554/img1982q.jpg"
width="400" border="0"/></a><br>
<a href="http://img7.imageshack.us/i/img1984i.jpg/" target="_blank"><img src="http://img7.imageshack.us/img7/3311/img1984i.jpg" width="400"
border="0"/></a><br>
<a href="http://img689.imageshack.us/i/img1985fi.jpg/" target="_blank"><img src="http://img689.imageshack.us/img689/3245/img1985fi.jpg"
width="400" border="0"/></a><br>
<a href="http://img543.imageshack.us/i/img1986d.jpg/" target="_blank"><img src="http://img543.imageshack.us/img543/9039/img1986d.jpg"
width="400" border="0"/></a><br>
<a href="http://img194.imageshack.us/i/img1989cc.jpg/" target="_blank"><img src="http://img194.imageshack.us/img194/1097/img1989cc.jpg"
width="400" border="0"/></a><br>
<a href="http://img522.imageshack.us/i/img1990x.jpg/" target="_blank"><img src="http://img522.imageshack.us/img522/9248/img1990x.jpg"
width="400" border="0"/></a><br>
<a href="http://img806.imageshack.us/i/img1996c.jpg/" target="_blank"><img src="http://img806.imageshack.us/img806/5299/img1996c.jpg"
width="400" border="0"/></a><br>
<a href="http://img560.imageshack.us/i/img1998s.jpg/" target="_blank"><img src="http://img560.imageshack.us/img560/2060/img1998s.jpg"
width="400" border="0"/></a><br>
<a href="http://img832.imageshack.us/i/img2000rv.jpg/" target="_blank"><img src="http://img832.imageshack.us/img832/1438/img2000rv.jpg"
width="400" border="0"/></a><br>
<a href="http://img233.imageshack.us/i/img2001z.jpg/" target="_blank"><img src="http://img233.imageshack.us/img233/7122/img2001z.jpg"
width="400" border="0"/></a><br>
<a href="http://img36.imageshack.us/i/img2003fx.jpg/" target="_blank"><img src="http://img36.imageshack.us/img36/8210/img2003fx.jpg"
width="400" border="0"/></a><br>
<a href="http://img189.imageshack.us/i/img2024jc.jpg/" target="_blank"><img src="http://img189.imageshack.us/img189/7891/img2024jc.jpg"
width="400" border="0"/></a><br>
<a href="http://img18.imageshack.us/i/img2026q.jpg/" target="_blank"><img src="http://img18.imageshack.us/img18/7384/img2026q.jpg"
width="400" border="0"/></a><br>
<a href="http://img10.imageshack.us/i/img2027qr.jpg/" target="_blank"><img src="http://img10.imageshack.us/img10/2182/img2027qr.jpg"
width="400" border="0"/></a><br>
<a href="http://img849.imageshack.us/i/img2028t.jpg/" target="_blank"><img src="http://img849.imageshack.us/img849/8962/img2028t.jpg"
width="400" border="0"/></a><br>
<a href="http://img577.imageshack.us/i/img2029f.jpg/" target="_blank"><img src="http://img577.imageshack.us/img577/7792/img2029f.jpg"
width="400" border="0"/></a><br>
<a href="http://img707.imageshack.us/i/img2030xs.jpg/" target="_blank"><img src="http://img707.imageshack.us/img707/3137/img2030xs.jpg"
width="400" border="0"/></a><br>
<a href="http://img684.imageshack.us/i/img2031fy.jpg/" target="_blank"><img src="http://img684.imageshack.us/img684/3905/img2031fy.jpg"
width="400" border="0"/></a><br>
<a href="http://img194.imageshack.us/i/img2032wp.jpg/" target="_blank"><img src="http://img194.imageshack.us/img194/7366/img2032wp.jpg"
width="400" border="0"/></a><br>
<a href="http://img20.imageshack.us/i/img2033u.jpg/" target="_blank"><img src="http://img20.imageshack.us/img20/5513/img2033u.jpg"
width="400" border="0"/></a><br>
<a href="http://img706.imageshack.us/i/img2034aq.jpg/" target="_blank"><img src="http://img706.imageshack.us/img706/5606/img2034aq.jpg"
width="400" border="0"/></a><br>
<a href="http://img64.imageshack.us/i/img2035f.jpg/" target="_blank"><img src="http://img64.imageshack.us/img64/3196/img2035f.jpg"
width="400" border="0"/></a><br>
<a href="http://img542.imageshack.us/i/img2038j.jpg/" target="_blank"><img src="http://img542.imageshack.us/img542/2969/img2038j.jpg"
width="400" border="0"/></a><br>
<a href="http://img713.imageshack.us/i/img2039r.jpg/" target="_blank"><img src="http://img713.imageshack.us/img713/6200/img2039r.jpg"
width="400" border="0"/></a><br>
<a href="http://img267.imageshack.us/i/img2040x.jpg/" target="_blank"><img src="http://img267.imageshack.us/img267/417/img2040x.jpg"
width="400" border="0"/></a><br>
<a href="http://img717.imageshack.us/i/img2042z.jpg/" target="_blank"><img src="http://img717.imageshack.us/img717/6412/img2042z.jpg"
width="400" border="0"/></a><br>
<a href="http://img715.imageshack.us/i/img2043yi.jpg/" target="_blank"><img src="http://img715.imageshack.us/img715/8638/img2043yi.jpg"
width="400" border="0"/></a><br>
<a href="http://img62.imageshack.us/i/img2044u.jpg/" target="_blank"><img src="http://img62.imageshack.us/img62/6889/img2044u.jpg"
width="400" border="0"/></a><br>
<a href="http://img13.imageshack.us/i/img2045d.jpg/" target="_blank"><img src="http://img13.imageshack.us/img13/1336/img2045d.jpg"
width="400" border="0"/></a><br>
<a href="http://img440.imageshack.us/i/img2046w.jpg/" target="_blank"><img src="http://img440.imageshack.us/img440/6695/img2046w.jpg"
width="400" border="0"/></a><br>
<a href="http://img706.imageshack.us/i/img2047lk.jpg/" target="_blank"><img src="http://img706.imageshack.us/img706/166/img2047lk.jpg"
width="400" border="0"/></a><br>
<a href="http://img42.imageshack.us/i/img2050v.jpg/" target="_blank"><img src="http://img42.imageshack.us/img42/3853/img2050v.jpg"
width="400" border="0"/></a><br>
<a href="http://img39.imageshack.us/i/img2051p.jpg/" target="_blank"><img src="http://img39.imageshack.us/img39/8188/img2051p.jpg"
width="400" border="0"/></a><br>
<a href="http://img94.imageshack.us/i/img2052g.jpg/" target="_blank"><img src="http://img94.imageshack.us/img94/5236/img2052g.jpg"
width="400" border="0"/></a><br>
<a href="http://img854.imageshack.us/i/img2053p.jpg/" target="_blank"><img src="http://img854.imageshack.us/img854/6359/img2053p.jpg"
width="400" border="0"/></a><br>
<a href="http://img688.imageshack.us/i/img2054et.jpg/" target="_blank"><img src="http://img688.imageshack.us/img688/197/img2054et.jpg"
width="400" border="0"/></a><br>
<a href="http://img197.imageshack.us/i/img2055sl.jpg/" target="_blank"><img src="http://img197.imageshack.us/img197/262/img2055sl.jpg"
width="400" border="0"/></a><br>
<a href="http://img705.imageshack.us/i/img2057os.jpg/" target="_blank"><img src="http://img705.imageshack.us/img705/9766/img2057os.jpg"
width="400" border="0"/></a><br>
<a href="http://img827.imageshack.us/i/img2071ql.jpg/" target="_blank"><img src="http://img827.imageshack.us/img827/3738/img2071ql.jpg"
width="400" border="0"/></a><br>
<a href="http://img714.imageshack.us/i/img2074j.jpg/" target="_blank"><img src="http://img714.imageshack.us/img714/9089/img2074j.jpg"
width="400" border="0"/></a><br>
<a href="http://img839.imageshack.us/i/img2075gz.jpg/" target="_blank"><img src="http://img839.imageshack.us/img839/6355/img2075gz.jpg"
width="400" border="0"/></a><br>
<a href="http://img809.imageshack.us/i/img2076yr.jpg/" target="_blank"><img src="http://img809.imageshack.us/img809/9866/img2076yr.jpg"
width="400" border="0"/></a><br>
<a href="http://img3.imageshack.us/i/img2077er.jpg/" target="_blank"><img src="http://img3.imageshack.us/img3/2954/img2077er.jpg" width="400"
border="0"/></a><br>
<a href="http://img28.imageshack.us/i/img2078y.jpg/" target="_blank"><img src="http://img28.imageshack.us/img28/6692/img2078y.jpg"
width="400" border="0"/></a><br>
<a href="http://img846.imageshack.us/i/img2086po.jpg/" target="_blank"><img src="http://img846.imageshack.us/img846/8344/img2086po.jpg"
width="400" border="0"/></a><br>
[Edited on 27-12-2011 by peach]
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: reduced
image size(s)]
[Edited on 9.12.13 by bfesser]
|
|
|
| Pages:
1
..
25
26
27
28
29
..
43 |