| Pages:
1
..
38
39
40
41
42
43 |
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I wrote a reaction to the mistake. Acyl chloride should be prescribed instead of anhydride.
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
Working with ketene is madness in a home lab.
GB299342 looks interesting as sodium metaphosphates are commercially available OTC. Sodium hexametaphosphate is a common food additive (E452i) and
water softener, and sodium trimetaphosphate is used in construction. Calgon according to some sources is sodium polymetaphosphate
Na2[Na4PO3]6 but polycarboxylate to others. Still I've seen 1lb bags of pure sodium hexametaphosphate
offered for under 10 euros as a water softener.
The patent from 1927 claims "the production of acetic anhydride from a mixture of sodium metaphosphate, anhydrous sodium acetate, and infusorial earth
moistened with glacial acetic acid, the reaction being effected at 150-180°C". Sounds too good to be true, but it could work, and yield wouldn't
really be an issue considering the requirements. I've heard mixed results from a similar low-yielding process, heating sodium pyrosulfate with sodium
acetate.
sic transit gloria mundi
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
non-US patents can be found on espacenet
https://worldwide.espacenet.com/
https://worldwide.espacenet.com/patent/search?q=GB299342
https://worldwide.espacenet.com/patent/search/family/0017418...
go to "original document"
The patents says to take the mixture of Ac2O and GAA and use it as solvent for a fresh NaPO3/NaOAc batch, increasing the yield
of Ac2O recovered every time.
[Edited on 27-1-2021 by dicyanin]

sic transit gloria mundi
|
|
|
LeLaborantin
Harmless

Posts: 6
Registered: 25-1-2021
Location: Belgique
Member Is Offline
|
|
Interesting procedure
Hello,
I'm sorry if this has already been discussed. But isn't it possible to proceed in this way to produce Acetic anhydride?
It sounds like a pretty interesting procedure.
UV LED lamps are not expensive and TCCA is easy to obtain.
What do you think about it?
(Sorry for my English, I'm French-speaking Belgian. )
Attachment: MICF_Leonor_Pereira.pdf (1.5MB)
This file has been downloaded 496 times 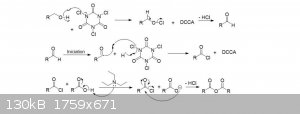
[Edited on 30-1-2021 by LeLaborantin]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
This method using TCCA and benzyl alcohol or benzaldehyde has been discussed, a few times in fact. It is probably the most OTC and easiest way to
benzoyl chloride.
However, caution is advised because one SM member reported an explosion when working up this rxn! The partially reduced DCCA and MCCA
byproducts can decompose to CO+N2+HCl.
A few alternatives might work better:
- Use AgBr or KICl4 as the halogenating agent instead.
- Add sodium benzoate upon rxn completion and extract benzoic anhydride with a nonpolar solvent. This way you convert cyanuric halo-acids to the salts
and they are retained in the polar phase, while Bz2O can be distilled or used in situ. Not easy because you can't use water to force a separation.
- Do the above, but with an N-halo monoimide, like succinimide or diformylimide. This means that only one salt will form, increasing the chance of
precipitation. In particular, since diformylimide has a small anion, it should have less soluble salts.
- Add excess acetic acid to the rxn mixture and try to remove AcCl by vacuum distillation at room temperature, so no heating is involved. Definitely
the best option if it works; probably need a good condenser.
- Do the above but distill AcCl at atmospheric pressure, around 80 C. The explosion reported by Johnny_Windchimes occurred at 180 C,
which is a decent margin of safety. Small scale only.
EDIT: Here is the post about why you should not try to distill BzCl from TCCA+BzH at atmospheric pressure ("boom!"):
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
[Edited on 1-2-2021 by clearly_not_atara]
[Edited on 1-2-2021 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Continuation of this locked thread - "Acetic Anhydride from Zinc Acetate?"
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
Here is the paper linked by pip.... Dunno what to make of it but my first thought is that it does come out of India. (big eye roll)
/CJ
Attachment: Synthesis of Acetic Anhydride by using Phosphorous Pentoxide, Sodium Acetate and Calcium Chloride as a Catalyst.pdf (1.5MB)
This file has been downloaded 449 times
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Yes! We have no bananas!
Phosphorus Pentoxide? No problem for me. Seemingly, I can buy as much as I want. For $20/LB. US Dollars. The problem is its high molecular WT. of
~280, and the limited amount of bang, you get for your buck.
Meaning the problem is the cost. Our fellow poster Len1, published a nice little procedure for producing POCl3 via Phosphorus Pentoxide.
POCl3 is a handy reagent, and hard to come by. And, so I suppose, worth it.
Using Phosphorus Pentoxide to make Acetic Anhydride, seems less than parsimonious.
It takes a significant amount of Phosphorus Pentoxide, to make a small amount of Ac2O.
Though I suppose, the spent Phosphorus Pentoxide can be utilized for some other purpose.
It cannot be easily regenerated. It's a one way street.
I did see a failed procedure on YouTube utilizing Phosphorus Pentoxide, but the experiment didn't have a robust design. They simply dripped Glacial
Acetic Acid onto Phosphorus Pentoxide solid, and produced some charred stuff.
Acetic Anhydride acquisition was not formerly a problem in the USA. There was a restriction on sales, but it only applied to very large amounts,
being transacted in international trade. A regular guy like me, could just walk into a supply company, and buy as much as I wanted.
A side note: Some of the Acetylations performed by Acetic Anhydride, might be performed by Isopropenyl Acetate. We have discussed this before, but
it has been a while.
To the best of my knowledge, Isopropenyl Acetate is unrestricted. So.... You might be able to just buy some.
Also, to the best of my knowledge, Isopropenyl Acetate isn't wildly toxic. But, that's just my knowledge. Does anybody have personal experience
working with it?
https://pubs.rsc.org/en/content/articlelanding/2012/gc/c2gc3...
https://www.sciencedirect.com/science/article/abs/pii/S00404...
[Edited on 11-2-2021 by zed]
[Edited on 11-2-2021 by zed]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Can't remember where but I think I remember a prep for isopropenyl acetate that involved bubbling MAP gas through glacial acetic acid.
zed, how are you getting P2O5 at that price? I don't think there are many brick and mortar stores that sell it.
[Edited on 2-11-2021 by njl]
|
|
|
LeLaborantin
Harmless

Posts: 6
Registered: 25-1-2021
Location: Belgique
Member Is Offline
|
|
Acetic acid and calcium Chloride
Good evening,
While looking a little bit on the internet, I came across this.
Has anyone ever tried it?
I'm putting the publication as an attachment.
I'll try this reaction soon
Edit : Oops it's already been put in a message before mine.
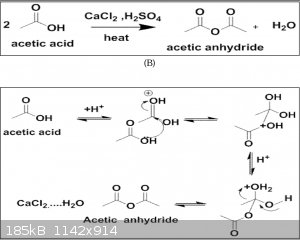
Attachment: doc.pdf (1.5MB)
This file has been downloaded 479 times
[Edited on 14-2-2021 by LeLaborantin]
|
|
|
xSJF1414
Harmless

Posts: 11
Registered: 19-9-2019
Member Is Offline
|
|
So I was just reading over acetic anhydride preparation on this came up; it seams way too good to be true but can someone verify this for me?
[Edited on 13-2-2021 by xSJF1414]

|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by zed  |
Also, to the best of my knowledge, Isopropenyl Acetate isn't wildly toxic. But, that's just my knowledge. Does anybody have personal experience
working with it?
|
I have experience in this method.
I even made Ac2O by this method,That is really easy
Just add AcOH and 2-3 drop Sulfuric acid and then distill it,first you get acetone and then Ac2O.
I even did this reaction with vinyl acetate and got same result but with less yield and with nasty acetaldehyde instead of acetone
[Edited on 17-2-2021 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Please elaborate...
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ketene is a deadly gas. Bad stuff.
Acetic Anhydride can be readily prepared via bubbling Ketene through Glacial Acetic Acid.
The procedure is pretty simple, though decidedly deadly if screwed up.
Acetic Anhydride, is a very basic and useful reagent, that for no particularly relevant reason, is quite difficult to purchase now-a-days.
Anyway.... Some folks may be able to acquire Iso-Propenyl Acetate. To the best of my knowledge, it is fairly uncommon, but it is unrestricted to
purchase.
Iso-Propenyl Acetate, is manufactured by the action of Ketene on Acetone. It might be considered an analog of Acetic Anhydride. Wherein unwilling
acetone, has been subjugated by powerful, super reactive, Ketene. Acetone thereby, being locked into it's Enol configuration.
Well, Acetone is a Ketone, and it doesn't like being locked up in an Enol configuration. Uncomfortable, and unstable. So.... Given a chance, it
will free itself from bondage, and send that Acetyl group, somewhere else.
This makes Isopropenyl Acetate, both an acetylating agent, and a potential source of Acetic Anhydride.
https://en.wikipedia.org/wiki/Isopropenyl_acetate
Thank you Waffles. I attempted this discussion about a decade ago, with Sauron present. The idea was not well received.
But, time has gone on, and Acetic Anhydride has become progressively more difficult to obtain.
Times change.
[Edited on 19-2-2021 by zed]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Read page 2923
REACTION WITH ACIDS AND ANHYDRIDE FORMATION
Attachment: Isopropenyl Acetate.pdf (703kB)
This file has been downloaded 540 times
From Vinyl acetate try DE503131 example 7
[Edited on 19-2-2021 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Thank you, Waffles! I knew something about the possibilities, in a theoretical way. Educated guessing.
But, you provided quite an expose'. Many more facts, than I had easy access to. 
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Wish I could find that old paper.
They were dehydrating acetic acid in a tube furnace.
It was over porous ceramic chips at 800 or 900 degrees. They got the reaction at lower temperatures by doping the chips with various chemicals,
(calcium phosphate?? some phosphate they prepared and cooked onto the ceramic at 800 or 900 degrees). the reaction started as low as 650 degrees.
The problem was the reaction gives you a very hot mixture of water, acetic acid, and acetic anhydride. Not a very stable concoction.
They cooled it fast in a quartz condenser in some cases. In others they used an inert carrier gas like ligroin to limit decomposition. The ligroin (or
whatever) was fed into the tube furnace along with the acetic acid in a large excess, and on condensation the anhydride wound up in the ligroin from
which the water could be separated. The solvent was then dried with some drying agent and distilled to get the anhydride.
It was an interesting paper but I neglected to copy it and the link I used is dead now.
Too bad, because I just picked up a 100 year old household appliance that will probably function as a tube furnace with some modification. It hits
760 degrees C inside the tube as it was built, and has hit 920 C with some insulation, but the insulation got fried. (Sone cheap Chinese stuff that
was supposed to be good to 1000 C. It came out looking like an overdone cannoli and part of it fused to the heating tube.)
Maybe the lack of a rheostat is making it harder on the insulation. Plug it in and it heats on full, and keeps doing so until you pull the plug. It
doesn't even have an on/off switch.
It's also one of the least safe appliances I've ever seen. Just putting your hand on it in the wrong place can give a shock with full house current.
Fortunately, it gets so damned hot in operation that you wouldn't want to touch it anyway.
https://www.picclickimg.com/d/l400/pict/173744823542_/Vintag...
|
|
|
Jenks
Hazard to Others
  
Posts: 129
Registered: 1-12-2019
Member Is Offline
|
|
Quote: Originally posted by LeLaborantin  | Good evening,
While looking a little bit on the internet, I came across this.
Has anyone ever tried it?
I'm putting the publication as an attachment.
I'll try this reaction soon
Edit : Oops it's already been put in a message before mine.
[Edited on 14-2-2021 by LeLaborantin] |
I notice that every time they get a good yield they use the least catalyst (e.g., CaCl2). It seems the secret to their success is to use the least
possible amount of "catalyst". I think the reason this is working is that they chill the acetic acid/sulfuric acid for 30 minutes before refluxing it.
Heating right away leads to charring right? But in theory, the hydrolysis of acetic anhydride is reversible - at room temperature, acetic anhydride
reacts with water to produce heat and acetic acid. The reverse of this, at room temperature, should absorb heat and produce acetic anhydride as water
is scrupulously absorbed. The problem is finding a sufficiently efficient drying agent. Assuming sulfuric acid will suffice, then equilibrating acetic
acid with sulfuric acid (or any other drying agent) at room temperature will produce acetic anhydride.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
see what you All were talking about how the ketene process is dangerous here is a guy doing the synthesis at 5ppm it's deadly compared to hydrogen
cyinide at 50 ppm.
https://youtu.be/g9sPlMkYEIw
Acetic anhydride using acetyl iodide
https://youtu.be/SGQ35JWq0eg
Acetic anhydride by nitrogen dioxide
https://youtu.be/gsVDuPjE9zY
Acetic anhydride by acetic acid and phosphorus pentoxide in DCM
https://youtu.be/Spd1RnRZbvk
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Recently a number of people on a sister forum have reported success with the acetonitrile/HCl method. It seems that the news has not yet reached
ScienceMadness, but I think it definitely seems like a major step forward in this quest. Scarecrow claims that the HCl gassing should
continue until the reaction mixture has nearly solidified (for a 1:1 AcOH/MeCN).
The product acetyl chloride is a bit nastier to handle than Ac2O, though.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
That's great to hear do share the process and link also what sister fourm?
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
It's not posted in this thread because the title is acetic anhydride. We hardly need posts and videos from anywhere.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Atara is referring to a thread on the vespiary by user Scarecrow called "Reliable OTC Acyl chloride synthesis (All C#)". I'm not sure what the policy
is for linking to the vespiary so I will edit in the link if asked.
Reflux condenser?? I barely know her!
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
BTW the original reference was posted by lugh and translated by Klute, in 07 here.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by njl  | | Atara is referring to a thread on the vespiary by user Scarecrow called "Reliable OTC Acyl chloride synthesis (All C#)". I'm not sure what the policy
is for linking to the vespiary so I will edit in the link if asked. |
Nothing wrong with it. Wack’s just
got a bug up his butt.
And to clarify, I would consider a discussion of acetyl chloride to be relevant to this thread, since you can easily convert it to acetic anhydride by
reacting it with glacial acetic acid. If there’s an easier way of making acetyl chloride than making acetic anhydride directly, then it may as well
be considered as a valid path to acetic anhydride.
[Edited on 4-30-2021 by Texium (zts16)]
|
|
|
Keras
National Hazard
   
Posts: 766
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Anyone has tried to reproduce the synthesis shown in this video which uses sodium acetate and S₂Cl₂, both being very easy to make?
|
|
|
| Pages:
1
..
38
39
40
41
42
43 |