| Pages:
1
2
3 |
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Nitrato Amine Nitrates: Nitrate ester explosives with reduced impact sensitivity
Michael A. Hiskey, Melvin J. Hatch, Jimmie C. Oxley
Propellants, Explosives, Pyrotechnics Volume 16, Issue 1, Date: February 1991, Pages: 40-42
Summary
The preparation and properties of several nitrato amine nitrates
are given along with calculated detonation velocities and measured
impact sensitivities. Comparison of these compounds with the high
performance explosive PETN is also given.
Attachment: Nitrato Amine Nitrates- Nitrate ester explosives with reduced impact sensitivity.pdf (324kB)
This file has been downloaded 2235 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Synthesis of nitroxyalkylammonium nitrates
L. B. Romanova, M. E. Ivanovo, D. A. Nesterenko and L. T. Eremenko
Russ. Chem. Bull., 1994, 43, 1207
Abstract
A method for O-nitration of amino alcohols with a non-detonating mixture of nitric acid with dichloromethane has been proposed. The target
crystalline products were precipitated from the reaction mixture by acetic anhydride. Key words: nitroxyalkylammonium nitrates; amino alcohols;
O-nitration; concentration limits of detonation; explosion-proof process.
Attachment: synthesis of nitroxyalkylammonium nitrates.pdf (153kB)
This file has been downloaded 1211 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Thanks solo.
<i>"...the target nitroxyalkyl nitrates are separated from the reaction mixture as oils that contain water formed during O-nitration, in
addition to the major compound. When acetic anhydride in an amount sufficient to bind the water is added to the reaction mixture, the target products
precipitate as crystalline solids and can be separated by ordinary filtration."</i>
Simple process, looked good until the requirement for acetic anhydride in the DCM/HNO3 nitration solution. Look for ways around the use of acetic
anhydride. Isolating the product may be its as simple as extracting the oil and sitting it in a dessicator.
Or, since the nitrate salt isnt so stable anyway it may be possible to form energetic derivatives of it directly from the oil. For example the nitroso
derivative ONC(CH2ONO2)3 by reacting with NaNO2, or the dichloramine Cl2NC(CH2ONO2)3 by reacting with NaOCl.
The chlorinated derivative, could possibly then be nitrated to form the nitramine (check US patent 6,603,018 for nitration of dichloramines to
nitramines).
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Many thanks for clearing up a longstanding question.
So Tris trinitrate can be made, but the nitrate salt of this is unstable, perhaps not surprisingly. Did you see anything about the stability of the
oil? It seems to me that getting the free amine from the unstable salt shouldnt be too difficult.
I'm adding the prep for this from the first paper that Solo attached:
| Quote: | To 35 ml of 99% HNO3 cooled to 0 deg C or below by means
of an ice-salt bath was slowly added 7.0 g of tris(hydroxymethyl)
aminomethane. Stirring and cooling were maintained
for 30 minutes after the final addition. The nitrating
liquor was slowly stirred into 200 ml of diethyl ether, at
10°C or below. The partially nitrated product immediately
precipitated and was suction filtered and washed with
CHCl3. Exhaustive nitration of the flaky white product was
accomplished by redissolving the entire amount in 20 ml of
99% HNO3 at - 20°C for 20 minutes. The product was
reprecipitated as before with diethyl ether, filtered, and
washed with ether and chloroform.The flaky white product
was dried under vacuum overnight. Upon weighing, the
product yielded 15.1 g (82%) with a m.p. of 110°C |
Sounds tedious. Here's the prep involving DCM (second paper from Solo)
| Quote: | A solution of
monoethanolamine (1.2 g) (substitute with the appropriate amount of Tris) in CH2C12 (5 mL) was added
dropwise over a period of 20 min to a cooled mixture of
HNO3 (3.8 g) and CH2C2 (25 mL). The temperature of the
bath was maintained in the range 0--5 deg C. Then the reaction
mixture was stirred at this temperature for 15 rain, acetic
anhydride (3 mL) was added dropwise, and the mixture was
stirred for an additional 15 min. The precipitate was separated
and dried in a vacuum desiccator over P205 to give 3.1 g
(91.2 %), m.p. 100--102 deg C. After recrystallization
from EtOH, the yield was
69--72 %, m.p. 102--103 deg C |
I wonder whether P2O5 or H2SO4 could simply be used. Or how about anhydrous MgSO4?
Another thought - perhaps it would be a good idea to derivatise the Tris before the nitration, making the nitration both easier, plus you can add on
goodies to the NH2.
However, what chemical groups are available that add well to amine groups, but leave hydroxyls untouched? CH2O would have been my initial choice, but
I assume that this reacts with the hydroxyls too. On the other hand, given the supposed prep. route to Tris, I suppose the beta carbon (which is
quaternary in Tris) needs to be free, so possibly attack by CH2O would be ok? - leaving the hydroxyls unaffected?
Alternatively, a Sandmeyer could be done, turning i.e. the NH2 to OH. I have a feel that Axt prepared this product, or rather the nitrated form,
already. Or was it from the combination of nitromethane and formaldehyde, whose product was nitrated, so slightly different as here we'd get
quaternary carbon linked to NO3, not NO2?
I doubt chlorination of the NH2 would work, again for the acidic character. It's bound to be unstable.
What other possibilities are there to play on the amine in simple reactions *before* nitration?
Here are some derivatives of Tris, all more or less common buffers used in the biological sciences. All look interesting, but I fear not all are going
to be useful for such purposes 
Tris

Bicine

bis (2-hydroxypropyl)amine

Bis-tris propane

Tricine

Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | | It seems to me that getting the free amine from the unstable salt shouldnt be too difficult. |
See last post on page two of this thread, theres problems with that.
| Quote: | | I wonder whether P2O5 or H2SO4 could simply be used. Or how about anhydrous MgSO4? |
I think you'd want an anhydride of a weak acid that soluble in DCM. P2O5 and H2SO4 aint you' end up with a slop under the DCM with a mixture of
nitrate/phosphate/sulphate salts. As with the MgSO4, it'd create problems in isolating the wanted product.
| Quote: | | Another thought - perhaps it would be a good idea to derivatise the Tris before the nitration, making the nitration both easier, plus you can add on
goodies to the NH2. |
I cant see any problems with CH2O, but the condensation product may be quite a mess, maybe not the best choice. By analogy with that of the synthesis
of nitraminoethanol nitrate, you could condense tris with ethylchlorocarbonate before nitration followed by ammonolysis to yield the nitramine (US
patent 2485855).
| Quote: | | Alternatively, a Sandmeyer could be done, turning i.e. the NH2 to OH. |
Doesn't that rely on an intermediate diazonium salt, tris in this case forming a stable C-nitroso product.
| Quote: | | I have a feel that Axt prepared this product, or rather the nitrated form, already. Or was it from the combination of nitromethane and formaldehyde.
|
Yeh that was NIBGTN via CH3NO2/CH2O then nitration.
| Quote: | | I doubt chlorination of the NH2 would work, again for the acidic character. It's bound to be unstable. |
Acidic character? not sure what you mean there, its a basic amine but also it doesnt necessarily need to be basic to form a chloramine. As long as its
a dilute solution the HNO3 shouldnt pose any problems, and actually would be needed to form the HOCl from NaOCl. I too dought its stability in the
practical sense of using it for an explosive as is, but that wasn't the point.
| Quote: | | What other possibilities are there to play on the amine in simple reactions *before* nitration? |
Ethylchlorocarbonate to form the urethane, diethyloxolate to form the oxamide, acetic anhydride to form the acetamide derivatives, all could possibly
be converted to the nitramine which I think is the most attractive target.
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
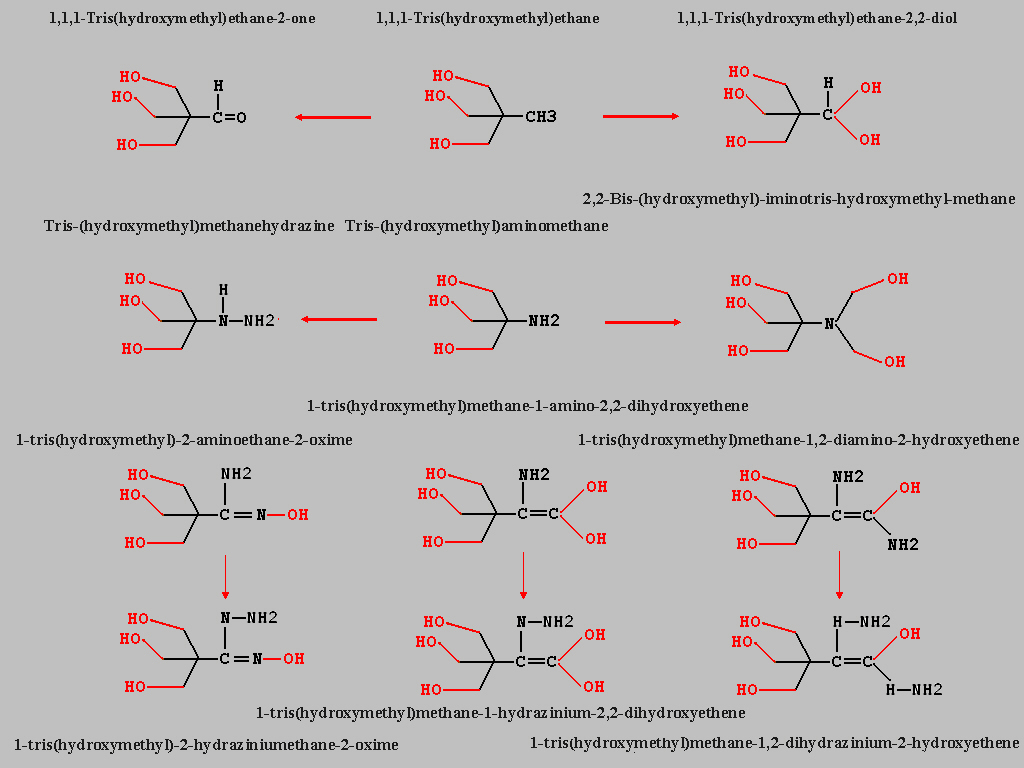
Thank for downloading the PDF`s. The drawings look
nice. Which drawing program do you use ? Maybe anyone
have a interested link to any useful drawing software. I
have designed a small picture to some new TRIS compounds
bevor nitration.
TRIS(hydroxymethyl)-2-one and TRIS(hydroxymethyl)-2,2-diol
which can prepared from TRIS(hydroxymethyl)ethane with
different chlorinations.
I would guess some other derivates like hydrazinium and
oxime compounds are useful, TRIS(hydroxymethyl)dihydraziniummethane and any ethylene compounds.
Some hints to practical issues may help. I`m not shure but
the sandmeyer reaction within the diazonium salt will form
some various results by a reaction within a amino group.
A example is, aniline in HBr/NaNO2 HBr/CuBr will give first the diazonium salt and then the bromobenzene and not a nitroso compound.
Sandmeyer Reaction
Might i can post a useful process to some of the new
compounds.
Excuse me, i`ve uploaded accidentally the wrong file. The
reviced verion of the picture is now uploaded.
The the correct names of the new mentioned compounds are:
1,1,1,-Tris(hydroxymethyl)ethane-2-one
1,1,1,-Tris(hydroxymethyl)ethane-2,2-diol
1,1,1,-Tris(hydroxymethyl)methanehydrazine
1-tris(hydroxymethyl)-2-aminoethane-2-oxime
1-tris(hydroxymethyl)methane-1-amino-2,2-dihydroxyethene
1-tris(hydroxymethyl)methane-1,2-diamino-2-hydroxyethene
1-tris(hydroxymethyl)-2-hydraziniumethane-2-oxime
1-tris(hydroxymethyl)methane-1-hydrazinium-2,2-dihydroxyethene
1-tris(hydroxymethyl)methane-1,2-dihydrazinium-2-hydroxyethene
BIS TRIS is availaible from chem suppliers (CAS-Nr. 6976-37-0), Tris(hydroxymethyl)methane and Tris(hydroxymethyl)aminomethane too.
(CAS-Nr. 77-85-0 and 77-86-1) The compounds should be easy to nitrate in different ratios with H2SO4 and HNO3.
[Edited on 13-11-2006 by Mason_Grand_ANNdrews]
[Edited on 14-11-2006 by Mason_Grand_ANNdrews]
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
I have there a nice suggestion at the moment to one of the
new energetic Tris compounds to my last uploaded drawing.
Sorry for the drawing errors within the nitramides, some
problems to convert the picture to a useful compressed
format. I want to produce tris-(hydroxymethy)-aminomethane by the way from tris-(hydroxymethy)-
nitromethane.
Im not shure if the reduction within a the hydroxyde will give
the correct product. Maight somone can test this out.
Tris-(hydroxymethy)-nitromethane: (C4H9NO5, 151,118
g/mol)
Prepare mixture of 61 g of nitromethane and 91 g of
paraformaldehyde in a 1000 mL flask. Place the flask to a
cold water bad and in samll portions add around 62 g of
dryed calcium hydroxide powder with rapid stirring. Hold the
temperature below 55 °C during the addition and continue
the stirring for an hour at 55 - 60 °C. Use a stirrer or a
hotplate if necessary. After that, stirring is continued for 2 or
3 hours, pour the mixture over a coarse filter or a sieve
to remove impurities and add enougt 5 - 10% hydrochloric
acid until the solution will become slight acidic. The water is
careful removed by vacuum concentration much as possible
and then the flask with filtered solution is placed in a salt ice
bad, around 500 mL of 95-98% sulfuric acid is added in
small portions and the product is separated and extracted
with several portions of methylene chloride and anhydrous
magnesium sulfate.
Tris-(hydroxymethy)-aminomethane: (C4H11NO3, 121,135 g/mol)
Have anyone a prcical idea to make this stuff by a useful
way. I don``t know the reduction of a alcohol will work
correct and do not form the tri-sodium salt, maybe the salt
can be neutralized with a dilute acid. I would advice that the
industrial manufacturing process is more than complex. One
of the chemical names of the compound is Aminomethylidine-
trimethanol. Maight the obtained product from synthesis 1 is
combined with 270g of tin powder, over a peroid of one hour
510 mL 25% HCl is stirred in, the temperature is hold below
60 °C and the mixture is than refluxed for one hour. After
that, a solution of 450 g sodium hydroxide in 600 mL water
in combined with the first, stirred a while until all is reacted
and the product is then obtained by vaporizing the water
and distilled above 173 °C. Maight a separation exactly as synthesis 1
will work. I`m not shure, but it is possible the obtained
product is tris-(hydroxymethylsodium)-aminomethane.
Does someone have a idea to converte the sodium salt back
into the hydroxymethyl ?
[Edited on 8-12-2006 by Mason_Grand_ANNdrews]
|
|
|
CD-ROM-LAUFWERK
Harmless

Posts: 30
Registered: 23-4-2005
Member Is Offline
Mood: No Mood
|
|
the ''U.S. Army Research and Development Command Encyclopedia of Explosives and Related Items CD-ROM.''
CD1, PDF nr. 1 (''VOLUME01''), page A132 left mid, says that both Bis(hydroxymethyl)methylaminomethan-dinitrat and
Tris(hydroxymethyl)aminomethan-trinitrat are unstable even at room temp, ref: H. A. Aaronson, P ATR 1412(1942)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
A very interesting possibility concerning TRIS has been noted by Axt in the pentryl thread
http://www.sciencemadness.org/talk/viewthread.php?tid=8027&a...
I haven't reread the PEP articles above to see if this may already have been attempted , to condense TRIS with
dinitrochlorobenzene , and then to further nitrate the
product of that condensation .
Something tells me Axt may be looking into this possible
route for a " heptryl " 
[Edited on 25-4-2007 by Rosco Bodine]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Certainly sounds like an interesting project!
Question is, will the free NH2 of Tris interfere with the coupling to dinitrochlorobenzene?
If it works of course, then there's a nice NH2 for further derivatisation!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I'll be optimistic about this proposed condensation of TRIS
with dinitrochlorobenzene , and say it should work under the same conditions as for ethanolamine .
Why let negative vibes sabotage theory in advance of an experiment .....making it sure to fail ?
Anyway the reaction looks good on paper and in theory
to me .
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Maybe I missed this, you are saying that the condensation with ethanolamine has been demonstrated? Where? Sorry for overlooking this if that's the
case.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The condensation of ethanolamine with DNCB has been done .
It produces the precursor for pentryl .
In the pentryl thread we were talking about other
possible condensations of polyhydric alcohols with DNCB ,
and of course the amino-polyhydric alcohols ( like TRIS ) come into consideration there also ,
as being even more likely
condensates , due to the reactivity of the amino towards
the chlorine .....which is like a magnet , and should also
at the same time simplify the solvent requirement for the condensation reaction .
[Edited on 25-4-2007 by Rosco Bodine]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Yes you are absolutely correct. I only looked at the more recent posts, on the matter of ethyleneglycol, but not on the one of ethanolamine.
Yes, this should work very very nicely!
Axt's indeed got a project there!
So you are thinking of the following compound
DNB-NH(NO2)C(CH2NO3)3 if I am not mistaken?
I viewed the reaction according to Nicodems scheme in the pentryl thread - which is, the OH being coupled to the benzene ring.
I was wondering whether a triply substituted Tris would occur, i.e.
H2N-C(CH2O[DNB])3, similar to the condensation found with ethyleneglycol.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Actually the ring gets one more aromatic nitro during the
side chain nitration , and becomes a trinitrobenzene ring
along with the side chain you show there . So just change that first D to a T and you got it 
Basically the end nitration product " heptryl " would have
the same structure as pentryl , with an extra pair of
nitroester groups in the side chain .
There's probably a series of aminopolyols which would
react accordingly with the DNCB so this could be a general reaction .
Concerning the possible condensation occuring with both of the ring chlorines on opposite sides in the case of para-DCB ,
yeah that same idea was discussed for ethanolamine earlier in the thread , and Nicodem didn't seem to be optimistic
about that reaction . However , it might or might not work .
I am not sure that Nicodem is the last word on this , even though I do value his input . These theoretical structures
are definitely in the realm of " test it and see what happens "
since theory is not perfect for predicting surprising results .
And what could be the eventuality is that the third ring nitro
entering may not occur. But this may not be significant , given
the addition of a second side chain which brings 3 nitroester
plus 1 nitroamino groups . If such a condensation of TRIS with both chlorines of para-DCB
did produce a symmetrical
aromatic di-alcohol of that sort which could be nitrated fully ,
it would have ten , 10 nitro groups ! ,
even in the absence of one TNB ring nitro which would have made it 11 ,
so there isn't much of a loss of energy there to be of concern .
Update :
I found an old post by Polverone where he tested paradichlorobenzene for reactivity of its chlorines ,
and it appears certain that nothing is going react
under any ordinary conditions , and that would include condensation reactions . So I have pretty well given
up on paradichlorobenzene , with regards to any reactivity
involving the chlorines anyway , for any lab syntheses .
Of course the discussion speculating about reactions for
para is something separate from the proven reactivity of dinitrochlorobenzene .
The proposition of condensing TRIS with DNCB still seems
to have validity .
I would say that the possiblity of alternate substitution
on both the hydrogens of the amino of TRIS does exist ,
to form a bis-dinitrophenyl condensate , similarly as occurs for ethanolamine condensing with DNCB .
But I have no idea what is the percentage probability
or distribution of either of the two possible products .
[Edited on 26-4-2007 by Rosco Bodine]
|
|
|
Explosci
Harmless

Posts: 9
Registered: 20-4-2012
Member Is Offline
Mood: www.explosci.com
|
|
Condensing tris with diethyl oxalate followed by nitration gives an explosive that is comparable with PETN
|
|
|
Maniak
Harmless

Posts: 45
Registered: 26-6-2004
Member Is Offline
Mood: No Mood
|
|
You probably just forgot to append reference for this statement so I'll do that for who's interested in details:
Thomas M. Klapötke and Norbert T. Mayr: Comparison of explosive performance and sensitivity of N,N'-bis(tris(nitratomethyl)methyl)oxamide with
PETN, In proceedings of NTREM 2007, Czech Republic, Pardubice, 2007, p. 773-782
PS: you've got nice blog
|
|
|
Explosci
Harmless

Posts: 9
Registered: 20-4-2012
Member Is Offline
Mood: www.explosci.com
|
|
Bingo, that is it.
|
|
|
| Pages:
1
2
3 |