Aqua-regia1
Harmless

Posts: 33
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
synthesis of 3-ethylamino-p-cresol
Hey everyone.
I have to to produce 3-ethylamino-p-cresol
Does anyone know a coupling way to do it?
(I don't have autoclave)
Thanks
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Please state your starting materials, and, define your target a little better.
Do you mean (from left) A, B or C?r other?
Most likely A I guess, correct me if I am wrong.
So are you permitted to select your own starting materials?
If so then 2,4-diaminotoluene would be good. You could alkylate the 2-amino to N-ethylamino, then diazotize the 4-amino and hydrolyze to the phenol.
Voila.
Or start with 2-amino-4-nitrotoluene. Alkylate the amine (unambiguously now), reduce the nitro to amine and diazotize to the phenol.
Many other possible routes depending on starting material. m-phenylenediamine for example. But then you will have to alkylate the ring ortho to one of
the amines. A little trickier.
[Edited on 20-10-2007 by Sauron]

Sic gorgeamus a los subjectatus nunc.
|
|
|
Aqua-regia1
Harmless

Posts: 33
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
Hy,
first sorry about my bad english! So, that's what i want to get at:
[img] [/img]
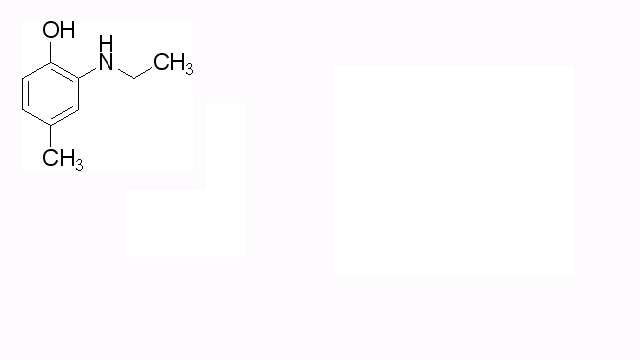
|
|
|
Aqua-regia1
Harmless

Posts: 33
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
I have / can synthetise the following precusors: 3-amino-4-hydroxytoluene, 3-bromo-4-aminotoluene, 2-amino-4-nitrotoluene, 2,4-diaminotoluene. How
can I get through to ethylamino group?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Ah. I believe cresols are numbered as methylphenols and not as hydroxytoluenes. But never mind.
You still have not specified your starting materials, and without doing so, I can't give you a single prep, I'd have to give you dozens.
Your target is 2-ethylamino-4-methylphenol (by IUPAC)
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Aqua-regia1
Does anyone know a coupling way to do it?
|
I can't help asking why would someone want to use a coupling reaction for that target when conventional chemistry is the only rational choice?
Mingling with organometalic chemistry when not needed is absurd, especially since you would have to use N- and O- protection group
chemistry and dry techniques. Is there a particular reason for preferring such a method? I'm very curious about the reasons.
With conventional chemistry you can prepare your target in as little as three steps from the commercially available m-aminophenol. Actually,
if you look at the more exotic chemical sellers, you can even find 3-amino-p-cresol and get it over in two steps. If you would have to use a
coupling reaction to introduce that methyl group, it would not be in less than something like 6 to 10 steps, including very expensive reagents and
catalysts. It is as absurd as asking how to prepare cyclohexene using alkene metathesis reaction.
[Edited on 20/10/2007 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Possible N-alkylating reagents
Iodoethane
Diethyl sulfate
Triethyl phosphite
Use your 3-amino-4-nitrotoluene.
Alkylate the amino. Be careful, alkylating agents are nasty.
Reduce the nitro (sodium dithionite/sodium carbonate methos)
Diazotize the primary amine and conver to phenol. You are done. Work up and purify.
[Edited on 20-10-2007 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
@Nicodem, no one seriously proposed a starting material not already containing the methyl group.
The alkylation he is concerned about is the N-ethylation.
Now that he has specified a set of precursors to choose from, an unambiguous short route is obvious as I outlined.
He was just stuck on how to add an ethyl to an aniline function.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
He explicitly mentioned he would prefer a coupling reaction. There are basically two kind of new bond creation in organic chemistry that are called
couplings: the C-C couplings (Suzuki, Heck, Negishi, etc.) and the acid with amine couplings. I assumed he wanted to use a coupling reaction for
introducing the methyl group, but then again I'm not so sure anymore. It is because that would be so irrational that I can't help about being
extremely curious.
|
|
|
Aqua-regia1
Harmless

Posts: 33
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
The name range in http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALD... (not IUPAC)
My plan was an accomplished fact : 3-Bomo-4-Methyl -phenol as precursor, was treated with Et-NH2 HCl + piridine + Cu2Cl2 + H2O (katalisator) in
130-150C degree (without autoclav)with very intensive condenser. This way attempt was barren of results. Any others idea?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
@Nicodem, it seems there is a language barrier at work. He keeps apologizing for bad English so I do not think you can count on his meaning of
coupling reaction being formation of a C-C bond. Read his last post. He tried to replace aryl Br with a primary amine (failed.)
@Aqua, see my post above, you said you have or can build, 3-amino-4-hydroxytoluene. Use that. Alkylate the aniline group with iodoethane (ethyl
iodide). diethyl sulfate or triethyl phosphite. One step.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
3-bromo-4-methylphenol has the bromine ortho to the methyl, but the target you sketched, has the ethylamine group (that you wanted to exchange with
Br) ortho to the phenol.
I think you are confused.
Your target is 2-amino-4-methylphenol, not 3-amino, is it not?
Sic gorgeamus a los subjectatus nunc.
|
|
|
Aqua-regia1
Harmless

Posts: 33
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
Thank you Sauron. I will alkylate the aniline group with iodethane. It is really one step. You came to realize that hard to understand my.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Sigma-Aldrich fucked up with this one. p-Cresol is numbered from the phenol side!
And Sauron is right. There is no way you would have obtained your compound from "3-Bomo-4-Methyl –phenol".
OK Aqua-regia1, now we finally found out what your target is (something you could have determined in the first post anyway!). So by "coupling" you
actually meant the Ullmann reaction? You can forget about getting any results with using ethylamine hydrochloride in pyridine moreover without an
autoclave. You would need 2-bromo-4-methylphenol. And you would need ethylamine for that, not the hydrochloride, in several fold excess and you need
to contain it in the reaction vessel at >150°C. Search the forum for instructions on how to build/obtain an autoclave. Or better yet buy it or ask
someone to build you one. You also need to purge the autoclave with inert atmosphere since the product and the catalyst gets oxidized with O2. And
still there are little chances you would get the product.
Is there any reason why you don't use any of the more classical approaches? For example, nitrosating p-cresol (or nitrating if you find a procedure
that would gives you the mononitrated product - there exist some), followed by hydrogenation in the presence of acetic anhydride and reduction of the
amide with diborane (or other appropriate reducing reagents). Unfortunately, you can not use ethylating reagents like Sauron proposes since you can
not monoalkylate anilines (except in >5 times excess of the aniline and still you would obtain a low yield of the monoethylated one).
O-ethylation would also be quite a problem.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Agreed, @Nicodem, acetylating the amino group and then reducing the acetanilide will neatly dodge the overalkylation nugbear.
Someone at Sigma-Aldrich deserves a kick in the tush for causing all this confusion, but the structural drawings should have cleared it up sooner. I
don't know of anything clearer short of a Vulcan mind-meld.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Aqua-regia1
Harmless

Posts: 33
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
As is well known, various nitrophenolic compounds present thermal explosive hazards! Thus, I give up
|
|
|
Aqua-regia1
Harmless

Posts: 33
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
I give up my plan to synthetise 3-nitro-4-hydroxytoluene (reduction= 3-amino-4-hydroxytoluene).
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Nitration of p-cresol would give you (even if you managed to limit reaction to exclusively mononitration) a mixture of 2-nitro-4-methylphenol and
3-nitro-4-methylphenol, the first being what you want and the second needing to be removed and reducing your yield. My best guess is that your desired
product would be the major product as the -OH group is more activating than is -Me. You CAN select reagents and conditions to avoid dinitration. Only
the dinitration products or higher would present a hazard.
Anyway you do have viable alternatives.
You can purchase 3-amino-4-nitrotoluene, thus avoiding having to do any nitration. Acetylate the aniline function, reduce the acetanilide to
ethylamino, and reduce the nitro to amine. Diazotize that and convert to -OH. Piece of cake. Choose your weapons carefully and you might combine the
two reductions into a single step, making this only three steps, otherwise four.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Aqua-regia1
Harmless

Posts: 33
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
What's happen when I try nitration of acet-p-toluidine? I guess , it is a safe route. Than hydrolise to amine, next step: phenol, at least nitro
reduction. What is your opinion?
|
|
|
Aqua-regia1
Harmless

Posts: 33
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
Finally: alkylation the aniline group with iodoethane.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Aqua-regia1
As is well known, various nitrophenolic compounds present thermal explosive hazards! Thus, I give up |
This sounds like you are either insulting our inteligence or you are not a chemist at all.
| Quote: | Originally posted by Sauron
You can purchase 3-amino-4-nitrotoluene, thus avoiding having to do any nitration. |
I can't find it available among the chemical sellers. Are you sure it is on sale?
Anyway, 2-amino-4-methylphenol is available and not even expensive. Actually it is 20-times cheaper than 2-ethylamino-4-methylphenol, the target.
PS: No (or "practicaly no" - to be exact) 3-nitro-4-methylphenol forms during the nitration of p-cresol. The only side products are the
2,6-dinitro-p-cresol and some products arising from ipso atack on at the position where the methyl is. One recrystallization would
be probably enough if conditions for mononitration would be found. In any case there is still the option of nitrosation to be used if nitration fails.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
@aqua,
Nitrating N-acetyl-p-toluidine (p-methyl-acetanilide) will give 2-nitro-4-methyl-acetanilide all right, you will have to avoid dinatration (2,6). Or
purify.
Then you must liberate the amine, diazotize and convert to phenol, only then reduce the nitro group and mono-alkylate. Monoalkylation is not easy, the
reaction favors dialkylation.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Aqua-regia1
Harmless

Posts: 33
Registered: 9-12-2006
Member Is Offline
Mood: No Mood
|
|
Good day Sauron!
I will isolate soon the 2-amino-4-methylphenol. What advice do you can give my, for a useful, and effektive monoalkylation. ( to become an good yield
for my endproduct) Thx: Aqua
|
|
|