Filemon
Hazard to Others
  
Posts: 126
Registered: 26-4-2006
Member Is Offline
Mood: No Mood
|
|
depolymerization PET
Hi!
Which it is best way depolymerization PET? I have proven with NaOH, it doesn't seem to have made effect. It should attack it slowly. But neither I am
sure to 100% the plastic is PET.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I really dislike the arrogance of unnecessary chemistry abbreviations. Some names are so long and unwieldy that abbreviations seem appropriate. I
don't know what PET is so I can't say in this case. I assume you're non-English-speaking? and you're saying you tried to deploymerize something
abbreviated as "PET" with NaOH and that failed?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ chemrox
Not do much plastic recycling then!!!!
PET (Polyethylene Terephthalate) is code No.1. It's the main plastic soft drink bottles are made of, sometimes also abbreviated PETE.
Regards, Xenoid
[Edited on 3-11-2007 by Xenoid]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Filemon
Which it is best way depolymerization PET? I have proven with NaOH, it doesn't seem to have made effect. It should attack it slowly. But neither I am
sure to 100% the plastic is PET. |
Hot concentrate aqueous NaOH or KOH will do it, 90 to 180 C for 15 minutes to several hours. See U.S. Pat. No. 3,544,622 for example.
Aqueous ammonia under pressure at 200 C also works, but not the sort of thing done in home labs. This gives the di-ammonium salt of terephthalic acid.
A mixture of PET, 50-50 water-ethylene glycol, and Na2CO3 will also work. Heat slowly with stirring to 150 to 170 C over an hour or so, use an air
condenser to reflux the glycol. As the water boils off and the carbonate decomposes the PET dissolves in the glycol and is hydrolysed. After cooling
hot water is added and the mix filtered to remove unreacted PET. IF the filtered solution is treated with CO2 the terephthalic acid precipitates out
of solution, the aqueous solution or Na2CO3 and ethylene glycol can be reused.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I don't do any plastic recycling except my own through the local waste hauler but thanks for clearing that up. The Polyethylene, PE for those like my
engineer that like that sort of thing, recycling I've looked at was either mechanical or at a huge energy deficit making it unattractive. The
shredding, weaving, mixing and extruding on the other hand leads to all sorts of good products like shoes and building materials that are weatherproof
and almost indestructible but I'm way off topic aren't I?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
I thought PET stood for poly-ethylenetetramine? I imagine that there could be ambiguity, however if you say that it stands for the terephthalate then
I guess you know whet you are talking about.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Man, what planet are you on Antwain? googling "poly-ethylenetetramine" yields precisely zero results. "polyethylene tetramine" yields nine results,
but soon will have 10 when it picks up on this thread. 
PETE is a polyester of alternating units of terephthalic acid and ethylene glycol.
[Edited on 11-4-07 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
WHile I agree with Chemrox that "unnecessary abbreviations" - I'd call them idiotic acronyms - are a curse generally, I think I'll excuse Filemon in
this case because he give the clue "depolymierization" indicating the P means a polymer and hence a plastic. As Xenoid says, I.m surprised Chemrox
hadn't heard of it. Even I had.
However, many posts here do use abbreviatons, especially for organc substances. Many I just don't bother to read because I haven't the faintest idea
what they are talking about. It's extremely bad practice.
Use them but all means but at least define what they mean ONCE, as any decent technical literature does. I don't mind things like MeOH or Ac radical,
or common names like malonic acid for propanedioic acid.
If you have worked in several fields the same stupid acronyms can mean several different things. I can't be bothered to work out what, I just trashcan
the post and move on. Use an IUPAC or similar name, once. True, that can be confusing, like 1,3 propylenedione for carbon suboxide C3O2, O=C=C=C=O
These acronyms always made me think of the druggies and their acronyms for their foul products.
Der Alte
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
https://sciencemadness.org/talk/viewthread.php?tid=4952
and the pdf version
http://www.sciencemadness.org/member_publications/terephthal...
Which for some reason does not show up in the member publication section(as linked to on the homepage, not the subforum)
[Edited on 4-11-2007 by The_Davster]
|
|
|
Filemon
Hazard to Others
  
Posts: 126
Registered: 26-4-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemrox
I really dislike the arrogance of unnecessary chemistry abbreviations. Some names are so long and unwieldy that abbreviations seem appropriate. I
don't know what PET is so I can't say in this case. I assume you're non-English-speaking? and you're saying you tried to deploymerize something
abbreviated as "PET" with NaOH and that failed? |
You are right completely. Also i difficult to understand the meaning of the abbreviations.
I did not believe that I was a so hard depolymerize PET.
[Edited on 10-11-2007 by Filemon]
|
|
|
Filemon
Hazard to Others
  
Posts: 126
Registered: 26-4-2006
Member Is Offline
Mood: No Mood
|
|
I have read in wikipedia that the PET breaks down with the heat in acetaldehyde. Does it decompose in phthalic anhydride?
http://en.wikipedia.org/wiki/Polyethylene_terephthalate
[Edited on 10-11-2007 by Filemon]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The amount of acetaldehyde formed is small, it is more an issue of affecting flavours than a route to producing using amounts of compounds.
As it's terephthalic (para) acid, you're not going to get the ortho-phthalic acid (or anhydride) out of the decomposition.
PET is a condensation polymer, formed by elimination of part of the monomeric reactants (possibly in stages), simple depolymerization back to the
monomeric parents isn't going to happen. The hydrolytic depolymerization is slow in part because of the difficulty in mixing the reactants, the
surface of the polymer is exposed to reaction if it's not brought into solution and even then you get steric blockage.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
O=C=C=C=O is of course not 1,3-propylenedione
But "carbon suboxide" conveys nothing about the structure nor even the formula.
The IUPAC name is
Propa-1,2-diene-1,3-dione
or 1,2-propadiene-1,3-dione
PS
"1,3-propylenedione" would be O=CH-CH=C=O
or more properly propene-1,3-dione
Numbering the position of the pi bond servews no purpose because of symmetry.
[Edited on 11-11-2007 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Carbon suboxide, C3O2, is resonance-stabilized, the other canonical forms being:
(+)O[triplebond]C-C(-)=C=O <----> O=C=C(-)-C[triplebond]O(+) .
It is this resonance-stabilization that enables C3O2 and C5O2 to exist, but not C2O2 or C4O2.
It an evil-smelling, highly reactive, very poisonous gas (comparably to CO). Have you ever breathed some of it, Sauron? It would probably made a
"good" war gas (perish the thought - it is Armistice Day!).
It is the anhydride of malonic acid (I previously mistakenly wrote "maleic"), from which it can be made. Uses of it are by way of its ready ability to
undergo Diels-Alder additions.
[Edited on 11-11-07 by JohnWW]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The anhydride of maleic acid? I'm sorry, John, but that position is occupied.
[Edited on 11-11-2007 by Sauron]
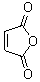
Sic gorgeamus a los subjectatus nunc.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Ya, so anhydrate that molecule one more and you get C3O2. It's the anhydride anhydride, duh. 
Tim
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
never mind i was thinking of polyethylene (recycling code 2) for thermal decomposition.
Chemkid
[Edited on 11-11-2007 by chemkid]
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
Der Alte said:
| Quote: | | 1,3 propylenedione for carbon suboxide C3O2, O=C=C=C=O |
And Sauron replied (correctly. Of course!):
| Quote: | | O=C=C=C=O is of course not 1,3-propylenedione |
Der Alte was probably under the influence of too many German high strength beers when he wrote that! Mea maxima culpa. Given propylene, -C=C-C- it’s
damned hard to see how to ‘diene’ this!
Quibble: given that propadiene has to be –C=C=C-, is 1,2 propadiene really necessary? Further, since it’s symmetrical, surely this says there is
only one dione? So is not propadiene dione sufficient to uniquely identify?
Nice to see you back in the saddle, Sauron, correcting us plebs.
@Filemon: Apologies for hijacking your thread. It happens.
With regard to polymers in general, I did a quick search on the Web for recycling of same. It appears that with one exception, all recycling is of
thermoplastics which are remelted to produce an inferior product containing a mixture of any original fillers – hence black or brown trash bags for
PE. Petrochemical companies do apparently use pyrolysis to provide and use some small radicals but true depolymerizations do not seem to exist in the
recycling industry. Hence they must be uneconomical, if not impractical.
Re: abbreviations for plastics, see www.matweb.com/reference/abbreviations.asp for a comprehesive list, including many I have never heard of.
Regards,
Der Alte
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
| Quote: | Originally posted by DerAlte
Quibble: given that propadiene has to be –C=C=C-, is 1,2 propadiene really necessary? Further, since it’s symmetrical, surely this says there is
only one dione? So is not propadiene dione sufficient to uniquely identify? |
You'd think so. But there's also 2-propanone and 2-butanone, among many others. Why these verbose names persist is beyond me.
| Quote: | | Petrochemical companies do apparently use pyrolysis to provide and use some small radicals but true depolymerizations do not seem to exist in the
recycling industry. Hence they must be uneconomical, if not impractical. |
This evening on Discovery they were showing the FutureCar miniseries. They mentioned a company which is turning shredder residue (namely, the
nonmetallic refuse leftover from chewing crushed automobiles into small pieces), which includes a variety of foam and plastic type materials, is
depolymerized in a reactor into an emulsion of water and fuel, supposedly similar to diesel.
Wikipedia probably refers to the company in question.
http://en.wikipedia.org/wiki/Thermal_depolymerization
Tim
|
|
|