Waffles
Hazard to Others
  
Posts: 196
Registered: 1-10-2006
Member Is Offline
Mood: No Mood
|
|
Digg new periodictable.com
http://digg.com/general_sciences/periodictable_com_Beautiful...
Take a look at the new site, masterfully redone by Theodore Gray. And yes, 24,000 decay chains all shown graphically  . And please digg it! . And please digg it!
\"…\'tis man\'s perdition to be safe, when for the truth he ought to die.\"
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
That's really nice!
|
|
|
Phosphor-ing
Hazard to Others
  
Posts: 244
Registered: 31-5-2006
Location: Deep South, USA
Member Is Offline
Mood: Inquisitive
|
|
I really like the spin option on the images of the elements.
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
I'd love the coffe table - but I don't have a spare £5G to spend on one. It's an element collection set in resin and made into a coffee table -
beautiful!
http://www.element-collection.com/html/coffee_table.html
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Here are some photos of my element collection (many samples are self made), it's not a full version but contains many interesting elements.
Na, K, Rb:
  
Cs, Be, Mg:
  
Ca, Sr, Ba:
  
Sc, Y, Ce:
  
Ti, Zr, Hf:
  
V, Nb, Ta:
  
Cr, Mo, W:
  
Mn, Re, Ni:
  
Co, Cu, Ag:
  
Au, Cd, Hg:
  
Ga, In, Tl:
  
Si, Sn, Pb:
  
P, Sb, Bi:
  
S, Se, Te:
  
Cl, Br, I:
  
B, Xe:
 
Nd, Sm, Gd:
  
Er, Tm, Lu:
  
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Is that actually liquid Xe? What's the critical temperature and pressure on that stuff?!
Tim
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
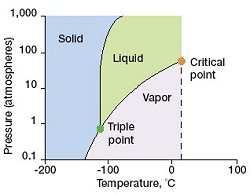
Xe phase diagram. Surprisingly hard to find more detailed ones.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by 12AX7
Is that actually liquid Xe? What's the critical temperature and pressure on that stuff?!
Tim |
Yes it is, pressure in ampoule is 72 atm, so then temperature is lower then 16C xenon liquifies, then sample is heated above this temperature xenon
goes to critical state.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Awesome!
What's it look like slightly warmer? Does the liquid disappear when you hold it in your hand too long? Does it become an homogeneous supercritical
fluid, sort of with the properties of both liquid and gas?
Tim
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by 12AX7
Awesome!
What's it look like slightly warmer? Does the liquid disappear when you hold it in your hand too long? Does it become an homogeneous supercritical
fluid, sort of with the properties of both liquid and gas?
Tim |
Then heated, border between liquid and gas becomes blurry and suddenly dissapears completely.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Do you make those ampoules Engager, or was it a friend of yours? I'm really impressed with them and I'd like to buy one.
It would be a nuisance to have to make my own and I don't have any pressurized xenon, nor do I want to shell out 1000 bucks to get 20 some liters of
it. I know we had a thread here about liquefying chlorine gas; easy enough with a lecture bottle, LN2, and some certain glass elements.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by Fleaker
Do you make those ampoules Engager, or was it a friend of yours? I'm really impressed with them and I'd like to buy one.
It would be a nuisance to have to make my own and I don't have any pressurized xenon, nor do I want to shell out 1000 bucks to get 20 some liters of
it. I know we had a thread here about liquefying chlorine gas; easy enough with a lecture bottle, LN2, and some certain glass elements.
|
Actualy this ampoule is not self made, i got it as exchange with guy who runs site www.periodictable.ru (remember to click GB flag at the top right corner to switch to english language); he works in Swiss, and as far as i know he
sells this ampoules on eBay or may be in some other place, you should contact him and discuss details.
[Edited on 18-12-2007 by Engager]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I also have quite a nice element collection already. I am still missing some of the most expensive metals, black phosphorus, uranium and rubidium.
http://woelen.scheikunde.net/science/chem/compounds/index2.h...
I especially like my halogen samples and the gas discharge tubes of most gaseous elements. I also have such a nice xenon sample, as mentioned by
engager. Just click on the Xe-element in the periodic table chart and scroll down to see detailed images of this sample. It really is beautiful.
|
|
|
contrived
Hazard to Self
 
Posts: 56
Registered: 9-3-2007
Location: Washington State
Member Is Offline
Mood: skeptical
|
|
Really nice period table plus plus plus! My new background.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Hey, that's a good idea. All allotropes of each element. Like C nanotubes and bucky balls.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|