| Pages:
1
..
19
20
21
22
23
..
27 |
z13123
Harmless

Posts: 4
Registered: 20-9-2014
Member Is Offline
Mood: No Mood
|
|
Thanks for the reply. I made sure it was neutral by testing it with pH paper. I'll try to detonate it ASAP and be more prepared next time. For some
reason, detonating it slipped my mind because I was too focused on synthesizing it... I'll make sure I have a better way to detonate it next time.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
That stump from a few posts ago, which was not removed, was blasted recently with a 400g charge of nitroglycerine powder containing 10% NG. The blast
was successful and the two pieces of the stump still in the ground are each attached by a single large root and can be wiggled by hand easily. A set
of come alongs will be able to pull them out by anchoring to a nearby tree. A higher velocity dynamite or a larger charge would have removed the stump
completely. I have found that once they are blown apart like this, however, that the remaining pieces are very easy to pull out of the ground.
You can see in the last picture the aluminum casing from the failed urea nitrate charge. It was actually a fairly difficult stump to blast because of
the very large roots which were very spread out and well anchored and with large holes between them for the explosive gases to escape through.
    
[Edited on 10-10-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
As to the idea of making small Nitroglycerin charges, I've found that most commonly people soak NG into Ammonium Nitrate or similar. Is it possible to
soak NG into a TNT mixture?
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
I don't see any reason it wouldn't work, a more brisant version of TNT, though I wouldn't hold it for a while. I imagine the mixed phase will end up
separating, liquid from solid. That is already a common problem with dynamite though, but more so with TNT. A fine sized TNT would help with this
but its waxy nature would make it slow at best to form a fine powder.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by Hawkguy  | | As to the idea of making small Nitroglycerin charges, I've found that most commonly people soak NG into Ammonium Nitrate or similar. Is it possible to
soak NG into a TNT mixture? |
You could certainly DO that- But it would not seem to make economic sense, nor to provide any advantage. Quite the reverse, it could well lead to a
lower stability and greater sensitivity than TNT has.
See the following for commercial practices and economic/engineering considerations related to nitroglycerin mixtures-
Tenney Davis: Chemistry of Powder & Explosives
Phokion Naoum: Nitroglycerin and Nitroglycerin Explosives
It was once common to use a mixture of various nitrotoluene byproducts of 2,4,6 TNT manufacture ("TNT oils") mixed into nitroglycerin to lower the
freezing point of dynamites- Pure TNT was not used, AFAIK. Making use of waste stream from another explosive manufacturing process to manufacture low
freezing dynamite, rather than spending additional money and time to make nitro glycol or nitro sugars as low freezing additions made economic sense
to manufacturers at the time.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
HB, I hope the windows to the house in the background held up. I can understand the reluctance to use anything larger then you did. That's mighty
close the the dwelling to be using HE.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Was just a little thump actually, nothing that would disturb even a close neighbor. The earth and stump absorb most of the energy/noise; unless of
course an excessively large charge is used, then it can be noisy. If that charge had been initiated above ground then there would have been one hell
of bang. Most people are surprised just how quiet a properly charged shot can actually be. Yes, I was being a bit extra careful though, because of the
proximity to buildings, etc.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
The stump pieces were in fact easily removed. A four wheel drive Toyota Tacoma was used to pull out the two remaining large pieces of the stump. Roots
are similar to guy-wires; when there is only one guy-wire the wood is no longer well anchored to the ground and can be easily removed. Also the blast
tends to heave the pieces at least a bit, removing tension from the guy-wires/roots, which also weakens the roots hold on the ground facilitating
removal.
  
[Edited on 27-10-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
synthesis large quantity nitroglycerin
Hi All,
I synthesized small amounts of NG (~5 gm) for several times using AN salt instead of nitric acid.
I am just asking if someone could synthesize large amounts of NG ? and what precautions he took into consideration?.
I know that NG is sensitive and dangerous but I am sure there are skillful guys here who could share their experience.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
There are many descriptions here of doing such things- Start by reading this whole thread, perhaps?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Hi Bert 
I already went through many posts here , maybe i dropped the correct ones.
I am really talking about large quantities  , something over 1 Liter. , something over 1 Liter.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
You want advice and pro tips on how to make over 1 l of nitroglycerin as a single batch.
I am going to have to formulate a proper response to that, as regards your safety and forum policy- that may take me a while. I do not type accurately
with tachycardia.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
I am just asking for sake of knowledge, i read some patents about machine that can do so but i wonder if it can be made in a lab or so.
I could make 200 mL of NG in my small lab but I never needed such amount.
so dont think i am crazy but it is very interesting to know if someone could manage that process before.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Of course it can be done, and relatively safely too, by people who are experienced. It was routinely made in ton quantities in batch processes
industrially from what I understand. The risk factor increases sharply with increasing batch size, however, and even the pros did have occasional
accidents. There is no way for someone experienced to write you a simple procedure that is going to account for every possible eventuality or give you
the situational awareness that they would have. I would advise that you stick with smaller batches and just make more of them if you need more NG. I
think the most I ever made was 250mL in a single batch. Cleared up any deficits in attention that I had, at least while performing the synthesis. 
Is making large batches really worth it to you? If it is, start small and develop a good working understanding of the process and the dangers
involved first.
The processes involved are relatively simple, but there are very real hazards.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Sometimes the way a question is asked shows the questioner has not yet done enough research.
Take a look at my post on this same page, see the links to two books in sciencemadness.org library.
Naoum' book details industrial equipment and reagents used for every scale- From bench procedure suited to students/material testing lab, pilot plants
and full scale factory layouts for 1,000's of kg. Along with historical development of such procedures and mention of not a few deadly accidents.
Tenney Davis gives lab bench scale synthesis and describes commercial production and handling, including continuous process machinery.
I warn you: Batch size in such reactions can not be scaled linearly with safety. If you try your 5ml process at 200 X size, you will likely experience
a runaway, and may well be injured or killed.
When scaled up:
Volume (and heat production!) goes up as a cube.
The surface area (area needed for heat to escape!!!) goes up as the square.
It is very possible to set yourself up with a batch size and reagent addition profile where no amount of external cooling will save you.
Note that even the smallest glycerin testing batch sized apparatus detailed in Naoum has circulating chilled water cooling? It is not there for swank.

[Edited on 9-1-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Nitroglycerine in Large Batches
It is very possible that I have made errors in the following calculations. If you see some, please let me know.
Here is a correction to calculated amount of ice:
Quote: Originally posted by Hennig Brand  | Just realized I messed up the calculation for the amount of ice needed on the last page of this thread. The amount of ice required was based on the
mass of glycerine nitrated, assuming complete nitration, and I calculated as if it was based on the amount of NG.
Quote: Originally posted by Hennig Brand  |
An ice water bath is an excellent way to cool a nitroglycerine nitration reaction mixture.
Water heat of fusion: 334J/g (334kJ/kg)
Mass of ice needed for cooling per gram of glycerine nitrated assuming no heat transfer to surroundings:
(1.43kJ/1g Glycerine) * (1kg Ice / 334 kJ) = 0.0043 kg Ice/1g Glycerine Nitrated (4.3g Ice/g glycerine nitrated)
Ice needed for synthesis of 1L of NG = 1000mL(1.6g/mL)(4.3g Ice/g glycerine) = 6880g of Ice (6.88kg of Ice)
This is assuming no heat transfer from the surroundings! In reality the amount of ice required could be much more.
|
Here is the correction:
An ice water bath is an excellent way to cool a nitroglycerine nitration reaction mixture.
Water heat of fusion: 334J/g (334kJ/kg)
Mass of ice needed for cooling per gram of glycerine nitrated assuming no heat transfer to surroundings:
(1.43kJ/1g Glycerine) * (1kg Ice / 334 kJ) = 0.0043 kg Ice/1g Glycerine Nitrated (4.3g Ice/g glycerine nitrated)
Ice needed for synthesis of 1L of NG = 1000mL(1.6g/mL) / (227.09 g/mol) * (92.09382 g/mol) * (4.3g Ice/g glycerine) = 2790g of Ice (2.79kg of
Ice)
This is assuming no heat transfer from the surroundings! In reality the amount of ice required could be much more.
What a difference a little slip can make! |
As Bert was saying volume increases much more quickly than the outside surface area of the vessel. A sphere is considered as an idealized
representation of a round bottom reaction flask, full of nitration mixture. In reality it would only be partially full and would have an open top and
very likely a flat bottom in most cases, but the following is just an approximation.
Volume of Sphere = 4/3 * pi * r^3
Surface Area of Sphere = 4 * pi * r^2
If the radius, or diameter, is doubled the volume of the spherical vessel increases by eight time (2^3 = 8), while the outer surface area only
increases by four times (2^2 = 4). If we increase the diameter by three times, we get 27 times the volume and only 9 times the outer surface area.
Since heat produced is directly proportional to the volume of the reaction mixture and in most amateur settings reaction cooling takes place through
the side walls of the reaction vessel, it is very important to make sure that adequate cooling is provided for.
I worked through the numbers a bit this morning, because I became interested myself.
The heat evolved in a nitration reaction includes not only the heat of nitration but also the heat of dilution. If the nitration reaction temperature
is allowed to rise too high, oxidation processes can start to take place which are very exothermic and can quickly lead to uncontrollable runaway
reactions. If this starts to happen the nitration mixture must be immediately drowned in cold water to stop the reaction. In small scale NG
nitrations, runaway reactions are normally fairly benign, but as the surface area to volume ratio of the reaction mixture gets smaller (larger batch)
a runaway reaction can be very dangerous often resulting in explosion if the reaction mixture is not promptly drowned in water.
Heat of Nitration
"According to information from different sources the heat of nitration of glycerol to trinitroglycerine varies from 120 to 170 kcal (ca. 502 to 711
kJ) per 1 kg of glycerine." (Urbanski Vol. 2 pg. 46) The higher value of 711 kJ / 1 kg of glycerine will be assumed, for the heat of nitration, as a
worst case type of scenario.
Assume starting with pure glycerine, 95% HNO3 and 95% H2SO4. Assume the following composition for the mixed acid:
HNO3....40%
H2SO4...55%
H2O.......5%
Assume 6.5 parts by mass nitration mixture per part of glycerine will be used.
Per 1g Glycerine:
Pre Nitration Numbers:
HNO3: 0.4(6.5g) = 2.60g
H2SO4: 0.55(6.5g) = 3.58g
H2O: 0.05(6.5g) = 0.33g
Total mass = 6.51g
Percent by weight of total acid: 95%
Percent HNO3 based on anhydrous acid: 42%
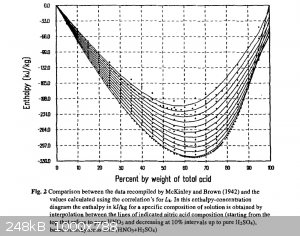
Enthalpy of mixed acid solution estimated from attached graph: -86kJ/kg
Post Nitration Numbers:
Per 1mole of glycerine nitrated, 3 moles of HNO3 are consumed and 3 moles of water are produced.
HNO3: 2.60g - 2.053g = 0.547g
H2SO4: 3.58g
H2O: 0.33g + 0.5864g = 0.916
Total mass = 5.04g
Percent by weight of total acid: 82%
Percent HNO3 based on anhydrous acid: 13%
Enthalpy of mixed acid solution estimated from attached graph: -254kJ/kg
Heat of Dilution
Heat of dilution = [5.04g / 1000g/kg * (-254kJ/kg)] - [6.51 / 1000g/kg * (-86kJ/kg)]
Heat of Dilution = -0.72kJ/1g Glycerine Nitrated
Total Heat Produced
Total Heat Produced = Heat of Nitration + Heat of Dilution of Mixed Acid
Total Heat Produced = -0.711kJ/1g Glycerine + -0.72kJ/1g Glycerine
Total Heat Produced = -1.43KJ/1g Glycerine
The nitration of glycerine is an extremely fast reaction, typically about 80% of the glycerine can be nitrated in less than one second according to
Urbanski Vol. 2. The associated section, discussing this, from Urbanki was snipped and made into a pdf which is attached. Assuming vigilant
temperature monitoring and good agitation/mixing, all that need be done to accurately control the temperature is to very carefully control the
addition rate (assuming the ambient temperature isn't a lot above room temperature). Good cooling makes the process much safer and certainly much
faster, however, since the reaction does produce a lot of heat which needs to be removed. As the batch size gets larger it quickly becomes impractical
and dangerous to not have powerful cooling in place.
Heat Capacity of the Reaction Mixture
The ability of the reaction mixture to absorb heat was examined.
From Engineeringtoolbox.com:
Specific Heats:
Sulfuric Acid: 1.38kJ/kg.K
Nitric Acid: 1.72 kJ/kg.K
Water: 4.19kJ/kg.K
Nitroglycerine: 1.7814kJ/kg.K (A Dictionary of Applied Chemistry)
The post reaction mixture was used in the calculation. Interactions between species were neglected, so as to provide a simple approximation of the
heat capacity of the mixture.
Average Heat Capacity of post reaction mixture = 0.547g/7.5089g(1.72kJ/kg.K) + 3.58g/7.5089g(1.38kJ/kg.K) + 0.916/7.5089g(4.19kJ/kg.K) +
2.4659g/7.5089g(1.7814kJ/kg.K)
Heat Capacity of post reaction mixture = 1.860996kJ/kg.K
Assuming no heat dissipation, for -1.43kJ heat produced per 1g glycerine:
deltH = m * Cp * deltT
Temperature Rise Assuming No Heat Dissipation = deltaT
deltaT = 1.43 kJ / [(1.860996kJ/kg.K) * (7.5089g/1000g/kg)]
deltaT = ca. 102K (102C)
Without adequate heat removal, the temperature of the reaction mixture could easily climb to very dangerous levels and this gets more
complicated/difficult as the reaction mixture gets larger.
An ice water bath is an excellent way to cool a nitroglycerine nitration reaction mixture.
Water heat of fusion: 334J/g (334kJ/kg)
Mass of ice needed for cooling per gram of glycerine nitrated assuming no heat transfer to surroundings:
(1.43kJ/1g Glycerine) * (1kg Ice / 334 kJ) = 0.0043 kg Ice/1g Glycerine Nitrated (4.3g Ice/g glycerine nitrated)
Ice needed for synthesis of 1L of NG = 1000mL(1.6g/mL)(4.3g Ice/g glycerine) = 6880g of Ice (6.88kg of Ice)
This is assuming no heat transfer from the surroundings! In reality the amount of ice required could be much more.
Very close temperature monitoring, glycerine addition control, excellent mixing and excellent cooling are all very important.
Good agitation/mixing keeps the temperature differential on either side of the vessel side wall at near maximum resulting in a near maximum
rate of heat transfer/rate of cooling. Good agitation also prevents localised hot spots which can/will result in exothermic decomposition (oxidation
processes) which could easily result in uncontrollable temperature rise/runaway.
Remember to get lots of ice before proceeding with the nitration, especially if it is a larger scale batch size.
Remember to keep the temperature below 30C. Actually, keeping the temperature well below 20C is safer and according to Urbanski these lower
temperatures normally produce higher yields as an added bonus for staying farther away from the dangerous, higher temperature, region.
Attachment: Nitroglycerine Nitration Reaction Rate from Urbanski Vol. 2.pdf (184kB)
This file has been downloaded 659 times
Attachment: Heat Effects Due To Dilution During Aromatic Nitrations By Mixed Acid In Batch Conditions.pdf (324kB)
This file has been downloaded 589 times
[Edited on 12-1-2015 by Hennig Brand]
[Edited on 14-2-2015 by Bert]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Anything over 100g glycerin input, you probably want a metal reaction vessel for better conduction. Naoum a page or two earlier describes a sheet Lead
vessel in an ice water bath for the same procedure shown with the fancy glassware above.
Moving upwards in batch size, you start seeing diagrams of cooling coils with circulating chilled water/brine and mixing paddles INSIDE the reaction
vessels... And jolly little tech notes about related issues: If the cooling coil starts leaking water into the concentrated nitrating acids, the heat
of dilution can easily lead to a runaway...
[Edited on 11-1-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I think it might be playing with fire a little bit, so to speak, but as long as the addition rate was carefully controlled, and the temperature was
closely monitored, I don't see any problems other than the process taking longer than if all the high powered cooling equipment were installed. With
good agitation glycerine doesn't hang around in the nitration mixture and then all react at once like sometimes happens with other nitrations.
Glycerine nitrates very quickly and completely even at very low temperatures. The reactions are very exothermic, however, and the temperature must be
watched like a hawk and additions must be slow and controlled. A big bucket of cold water must be handy to drown the reaction if controlling the
temperature becomes impossible (which should only happen if the process was rushed and/or there was a lack of cooling for the size of the reaction).
Obviously the bigger one goes the more dangerous it becomes, especially if there is a lack of understanding about
the process.
That glass apparatus above is very neat, but I don't think it is necessary for 200g or so of NG. It would probably be very convenient though. I have
made about a cup (~250mL) of NG before in a glass coffee pot. Other than my nerves it all went fine. It took a good couple of hours to add all the
glycerine, though, and I was stirring with a glass rod by hand (I now only swirl for safety). It wouldn't be very hard to rig up a Teflon paddle
driven by a cordless drill (powered by a mains fed power supply). With a good ice bath or salt ice bath and the Teflon paddle working vigorously
cooling should be fantastic through a thin walled glass round bottomed vessel.
Thermal Conduction Through Glass
I think you have a point about the different materials and thermal conductivity, but I don't think it is as much of an issue as you think at these
small scales.
Thermal Conductivities
Borosilicate Glass: 1.14w/m.K (camglassblowing.co.uk)
Stainless Steel: 16w/m.K (Engineeringtoolbox)
Lead: 35 w/m.K (" ")
Carbon Steel: 43w/m.K (" ")
Cast Iron: 55w/m.K (" ")
So yes, glass really stinks in the thermal conductivity department. However, I still don't think it is much of a problem at the scales we are
discussing.
From the reaction numbers in the last post, a 1L reaction mixture can accommodate the nitration of about 247g of glycerine. The spherical vessel will
be assumed to be twice as large as needed and only using half its surface area for cooling to the ice bath.
Q/t = [K * A * (TH - TC)] / d
Heat conduction/time = [Thermal conductivity * Area * (Temperature hot side - Temperature cold side)] / Thickness of sidewall
Assume that a 15C temperature differential is maintained. Just measured a coffee pot and it was 2mm thick or less; will use 2mm wall thickness.
Q/t = [1.14w/m.K * 0.03838m^2 * (25C - 10C)] / (0.002m)
Q/t = 328watts or 328J/s
Total Heat Produced = 247g Glycerine * (1.43kJ/1g Glycerine) = 353kJ
Time needed to transfer all heat using a 15C temperature differential = 353kJ / (0.328kJ/s) = 1077s or 17.9minutes
As long as the temperature differential was maintained at least 15C from one side of the glass sidewall to the other, 247g of glycerine could be
nitrated, producing about 500g of NG, and the heat produced could be transferred in less than 18 minutes.
I can see how this is maybe starting to push the limits, however, a much greater temperature differential could be maintained which would increase the
rate of heat transfer.
One of the important points to take note of; if a vessel with low conductivity, such as one made of glass, is used the ability to deal with a
temperature/energy surge is less.
[Edited on 12-1-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Hi Bert and Hennig,
This is a photo for the reaction vessel for NG at Nobel's first company.

for me it looks horrible  , I took the picture from BBC documentary video, here
is a link for this part : http://youtu.be/01pjt_K-94M?t=33m16s , I took the picture from BBC documentary video, here
is a link for this part : http://youtu.be/01pjt_K-94M?t=33m16s
It scare me when you talk about metal vessel for the reaction because we use conc Nitric or Sulfuric acid.
I always try to keep the reaction temperature between 0 to 5 degrees to be on the safe side.
I always use 5 times the amount of NG for synthesis as a rule of thumb.
it is really interesting to know how large scale production of NG works.
[Edited on 12-1-2015 by ecos]
[Edited on 12-1-2015 by ecos]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Ecos,
Please resize that image. It is way too big and it is messing up this page. I normally go 800 width, but I think you can go wider than that now and
still be fine. If you don't have a program to resize pictures you could try GIMP. It is what I have been using and it is free to download.
Nice picture, notice how the guy is sitting on a one legged stool. One way to help ensure the guy watching the thermometer stays alert.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Hi Hennig,
I modified the picture, it should be better now for your screen/
your last statement was mentioned in the youtube link i provided above , i wonder how a man can sleep while he is synthesizing NG  , he would loose his life for sure. , he would loose his life for sure.
what interest me is the size of the reaction vessel and the valves !!
I wonder how would he control the situation if the temperature starts to increase gradually without stopping , he would need a bigger container full
of ice or even KOH to stop the run out !
safety precautions is a point of interest here.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thanks. People often do become complacent or comfortable, after days or weeks, even when doing something as serious and important as monitoring the
temperature of a large scale batch NG production process. The flow of glycerine could be slowed or stopped completely depending on the temperature of
the reaction mixture. If things really became uncontrollable, there was a large reservoir of water below the reactor which the entire contents of the
reactor could be quickly dumped into by pulling a lever or some other control. From what I have read this was a common set-up to deal with runaway
reactions. Everything was normally designed to limit the damage in case of explosion as well. The buildings and the grounds around them were normally
designed to direct a blast up and limit the extent of the damage.
I have seen that documentary before, it is very good. I think I will watch it again. That image you posted was actually put up for discussion in one
of the classes I took at university a few years back.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Regarding the gelignite , do anybody have an instruction video for this?. I know the process but it would be more efficient to see a video.
i only found this video but it has no instructions : http://youtu.be/lcgSDrpWawQ
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
The following snip-it was taken from, "Lectures on Explosives" Third Edition (1902) by Willoughby Walke. There are other types of Gelignite as well
such as ammon gelignite.

I don't think I have seen a video, but I haven't really looked for one either. Basically the gelatine and dope are prepared separately and then the
two are well mixed. The gelatine can be much easier formed by using a bit of acetone, I have found, and then later allowing the acetone to evaporate.
The dope is well dried and powdered before mixing with the gelatine. It is a very simple process for the most part.
Apparently ammon gelignites replaced regular gelignite for the most part.
[Edited on 13-1-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
nice to know about this mixture, I always use this mixture:
90% NG
9% NC
1% Sodium bicarbonate as anti-acid, (Naoum used chalk in his book but i use sodium bicarbonate , not sure if i do the correct thing )
i hope if there is a video for the process 
|
|
|
| Pages:
1
..
19
20
21
22
23
..
27 |