| Pages:
1
..
20
21
22
23
24
..
27 |
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I think that mixture is normally referred to as blasting gelatine, though I see on the internet the terms gelignite and blasting gelatine often seem
to be used interchangeably. Gelignite is a high gelatine content dynamite with an absorbent dope of powdered nitrate and fuel from what I understand.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hennig Brand  | I think it might be playing with fire a little bit, so to speak, but as long as the addition rate was carefully controlled, and the temperature was
closely monitored, I don't see any problems other than the process taking longer than if all the high powered cooling equipment were installed. With
good agitation glycerine doesn't hang around in the nitration mixture and then all react at once like sometimes happens with other nitrations.
Glycerine nitrates very quickly and completely even at very low temperatures. The reactions are very exothermic, however, and the temperature must be
watched like a hawk and additions must be slow and controlled. A big bucket of cold water must be handy to drown the reaction if controlling the
temperature becomes impossible (which should only happen if the process was rushed and/or there was a lack of cooling for the size of the reaction).
Obviously the bigger one goes the more dangerous it becomes, especially if there is a lack of understanding about
the process.
That glass apparatus above is very neat, but I don't think it is necessary for 200g or so of NG. It would probably be very convenient though. I have
made about a cup (~250mL) of NG before in a glass coffee pot. Other than my nerves it all went fine. It took a good couple of hours to add all the
glycerine, though, and I was stirring with a glass rod by hand (I now only swirl for safety). It wouldn't be very hard to rig up a Teflon paddle
driven by a cordless drill (powered by a mains fed power supply). With a good ice bath or salt ice bath and the Teflon paddle working vigorously
cooling should be fantastic through a thin walled glass round bottomed vessel.
Thermal Conduction Through Glass
I think you have a point about the different materials and thermal conductivity, but I don't think it is as much of an issue as you think at these
small scales.
Thermal Conductivities
Borosilicate Glass: 1.14w/m.K (camglassblowing.co.uk)
Stainless Steel: 16w/m.K (Engineeringtoolbox)
Lead: 35 w/m.K (" ")
Carbon Steel: 43w/m.K (" ")
Cast Iron: 55w/m.K (" ")
So yes, glass really stinks in the thermal conductivity department. However, I still don't think it is much of a problem at the scales we are
discussing.
From the reaction numbers in the last post, a 1L reaction mixture can accommodate the nitration of about 247g of glycerine. The spherical vessel will
be assumed to be twice as large as needed and only using half its surface area for cooling to the ice bath.
Q/t = [K * A * (TH - TC)] / d
Heat conduction/time = [Thermal conductivity * Area * (Temperature hot side - Temperature cold side)] / Thickness of sidewall
Assume that a 15C temperature differential is maintained. Just measured a coffee pot and it was 2mm thick or less; will use 2mm wall thickness.
Q/t = [1.14w/m.K * 0.03838m^2 * (25C - 10C)] / (0.002m)
Q/t = 328watts or 328J/s
Total Heat Produced = 247g Glycerine * (1.43kJ/1g Glycerine) = 353kJ
Time needed to transfer all heat using a 15C temperature differential = 353kJ / (0.328kJ/s) = 1077s or 17.9minutes
As long as the temperature differential was maintained at least 15C from one side of the glass sidewall to the other, 247g of glycerine could be
nitrated, producing about 500g of NG, and the heat produced could be transferred in less than 18 minutes.
I can see how this is maybe starting to push the limits, however, a much greater temperature differential could be maintained which would increase the
rate of heat transfer.
One of the important points to take note of; if a vessel with low conductivity, such as one made of glass, is used the ability to deal with a
temperature/energy surge is less.
[Edited on 12-1-2015 by Hennig Brand] |
I used the wrong heat transfer equation above which does change the result by a small amount. The equation used was for a flat wall and what I should
have used was the equation for a sphere.
Q/t = (T1 - T2) / Rsph
where:
Rsph = (r2 - r1) / (4 * pi *r1 * r2 * k)
Rsph is the conductive resistance of the spherical layer
Using a 1L reaction mixture in a 2L spherical flask:
V = 4/3 * pi * r1^3
r1 = 0.0782 m
wall thickness = 2mm
r2 = 0.0802m
Rsph = (0.0802m - 0.0782m) / (4 * pi * 0.0802m * 0.0782m * 1.14w/m.K)
Rsph = 0.02226 C/w
Q/t = (25C - 10C) / (0.02226C/w)
Q/t = 673.8w
For half of surface area used:
Q/t = 673.8w/2 = 337w or 0.337 kJ/s
Time needed to transfer all heat using a 15C temperature differential = 353kJ / (0.337kJ/s) = 1047s or 17.5minutes
This actually improves the situation by a little bit.
In case anyone was interested here are the equations for one dimensional, steady state, heat conduction for a cylindrical layer as well:
Q/t = (T1 - T2) / Rcyl
Rcyl = ln(r2 - r1) / (2 * pi * k * L)
[Edited on 24-1-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hennig Brand  |
In case anyone was interested here are the equations for one dimensional, steady state, heat conduction for a cylindrical layer as well:
Q/t = (T1 - T2) / Rcyl
Rcyl = ln(r2 - r1) / (2 * pi * k * L)
|
That last equation should read:
Rcyl = ln(r2/r1) / (2 * pi * k * L)
Man I need to get my shit together.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Interesting equations for real
I am really sorry if my question would look simple : is it possible to provide an example for 1 L so we can know how to use these equations ?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by Hennig Brand  | | I think that mixture is normally referred to as blasting gelatine, though I see on the internet the terms gelignite and blasting gelatine often seem
to be used interchangeably. Gelignite is a high gelatine content dynamite with an absorbent dope of powdered nitrate and fuel from what I understand.
|
Blasting gelatine is usually just NG, enough NC to gell and bring OB to neutral and usually a small amount of antacid such as calcium carbonate. It's
high velocity, good for hard rock blasting, can cut steel & do similar jobs- But it tends to lose sensitivity to initiation over time.
Gelignites are active base dynamites where the NG has been pre gelatinized with a small amount of NC before mixing into the dope, whatever mixture of
powdered oxidizer(s)/fuel(s)/antacid are being used. They don't lose sensitivity in storage in my experience-
Amonium nitrate based gelignite is a nice versatile explosive, works fine in a range from 10% to 60%+ NG, depending on how fast you need it to shoot.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
No problem, r1 (inside radius) above was found by using 0.002m^3 (2L) for volume in the reaction flask/sphere volume equation. Outside
radius was found by adding wall thickness (0.002m (2mm) in the example) to the inside radius. It was assumed that the flask would be half full (1L
reaction mixture). Once the rate of heat transfer was found for the 2L flask/sphere it was divided by 2 to get the rate of heat transfer for the 1L
reaction mixture. In reality there could be some heating or cooling at the surface of the liquid reaction mixture, inside the flask, but what was done
should still be a close approximation of steady state heat transfer with a continuous 15C temperature differential. So the above was an example for a
1L reaction mixture.
Bert, that was more or less my understanding of blasting gelatin and gelignite as well.
[Edited on 27-1-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Volume & Area Equations for Partially Filled Spherical Vessels
Notice how above I calculated the rate of heat transfer for a full 2L flask and then simply divided by two for the assumed 1L contents. This is a
special case, since 1L would be half full and because of symmetry the surface area is also halved. Normally the spherical vessel used would not be
exactly half full, however, and the relationship between volume and area is not as simple.
Here are a couple of equations which could be useful to someone trying to perform heat transfer calculations based on a partially filled spherical
vessel/flask.
V = 1/3 * pi * H^2 * (3 * R - H)
A = 2 * pi * R * H
where:
V = Volume of contents in partially filled spherical vessel
A = Inside Surface Area of curved surface, next to contents, of partially filled spherical vessel
H = Height of contents in partially filled spherical vessel
R = Inner Radius of spherical vessel
Equations for other shapes can be found too, in texts or online.
[Edited on 28-1-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Hi Hennig,
I always go through your equations and got lost easily  , As I understand you try
to calculate the heat transfer or heat generation due adding NG with acids into ice ! , your first equations says we need ice with volume at least 6
times the volume of mixture (NG + acids) , As I understand you try
to calculate the heat transfer or heat generation due adding NG with acids into ice ! , your first equations says we need ice with volume at least 6
times the volume of mixture (NG + acids)
I didnt see any equations showing the required temp of ice+water!.
if someone used water with temp = 5 degrees instead of ice, would that be risky ?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
If you have ENOUGH cold water, and the reaction vessel configuration and rate of addition allow the heat to be removed, it will be possible to prevent
a run away. Determining in advance HOW MUCH ice and WHAT SHAPE/SIZE reaction vessel is what all the engineering math earlier was about. You only need
to keep the charge below someplace around 20 C, but colder gives more margin for error-
The key difference in starting with cold water: there is a substantial amount of energy required to MELT the ice. You're going to need substantially
MORE cold water than ice, even comparing liquid water at .001 ºC, to solid ice at -.001 ºC.
Water heat of fusion: 334J/g (334kJ/kg)
See area "B" of graph below:
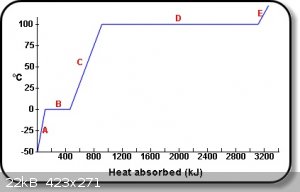
The diagram shows the uptake of heat by 1 kg of water, as it passes from ice at -50 ºC to steam at temperatures above 100 ºC, affects the
temperature of the sample.
E: Steam absorbs heat and thus increases its temperature.
D: Water boils and absorbs latent heat of vaporization.
C: Rise in temperature as liquid water absorbs heat.
B: Absorption of latent heat of fusion at 0 ºC,
A: Rise in temperature as ice absorbs heat.
from-http://www.physchem.co.za/Heat/Latent.htm
[Edited on 6-2-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
As Bert pointed out there is a phase change when ice changes to water, which absorbs a lot of heat/energy without temperature change. It takes about
the same amount of energy to melt a kg of ice as it does to raise the temperature of a kg of water from 0C to 80C. Heat transfer was through the glass
sidewall of the spherical vessel in the example. The ice was to go in the ice water bath surround which the heat of nitration and heat of dilution was
transferred to (through the glass sidewall).
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
ok , i got the message  thanks all thanks all
I always nitrate glycerin at 5 degrees, so it would be an advantage to cool it more before pouring it in the ice path.
they state in referencing "the mixture is added in one portion to crushed ice" or "the mixture was added to ice path in one shoot"
why they use the wording : "one portion or one shoot" ?
[Edited on 6-2-2015 by ecos]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
When and if I try a large batch such as was described by the above calculations, I will use a small submersible centrifugal fountain/pond pump to
circulate the ice water in the ice bath surrounding the nitration vessel (these can be purchased as table top fountains for 10 to 15 dollars). The
nitration mixture will be stirred with a Teflon paddle powered by drill. Keeping both the nitration mixture and ice bath well mixed will provide a
near maximum rate of heat transfer, since the temperature differential from one side of the reaction vessel sidewall to the other will be kept at near
maximum. Forced convection allows much greater rates of heat transfer than natural convection.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
A drill? Sounds kinda messy? Maybe you should just try a little motor scrapped from a toy or something...
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Would that be any less messy? Besides, drills have built-in speed control, and with the right equipment, the drill will be out of the path of the
acid.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Overhead stirrers are nice for stuff that bogs a magnet, or as in this case where a cooling/heating bath interferes. I have used a drill & hose
clamps etc. as well, trying to buy a dedicated outfit now just to not have to mess with speed control & mounting.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Overhead stirrers can also be run so that they don't contact the sides or bottom of the reaction vessel, which can be an advantage in safety with
friction sensitive materials.
I don't think there needs to be any mess. If there is significant splashing it likely means the stirrer is going too fast or the stirring paddle, or
other attachment, is not of the right type or some other mix-up. Cordless drills can be modified for accurate speed control easily, since they use a
DC motor. They can also be made to run from a mains fed power supply. Even regular corded drills normally have brushes, as far as I know, so they too
can be easily speed controlled by simply changing the voltage supplied to them or better yet using a triac type speed controller. I found a hobby
speed controller circuit here:
http://www.electroschematics.com/444/motor-speed-regulator-w...
"This triac-based 220V AC motor speed controller circuit is designed for controlling the speed of small household motors like drill machines. The
speed of the motor can be controlled by changing the setting of P1. The setting of P1 determines the phase of the trigger pulse that fires the triac.
The circuit incorporates a self-stabilizing technique that maintains the speed of the motor even when it is loaded.
220VAC Motor Speed Controller Schematic
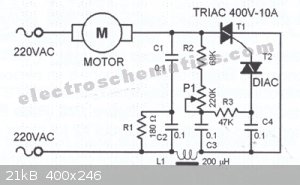
For example, when the motor of the drill machine is slowed down by the resistance of the drilled object, the counter-EMF of the motor also decreases.
This results to a voltage increase in R2-P1 and C3 causing the triac to be triggered earlier and the speed increases accordingly."
[Edited on 9-2-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Just realized I messed up the calculation for the amount of ice needed on the last page of this thread. The amount of ice required was based on the
mass of glycerine nitrated, assuming complete nitration, and I calculated as if it was based on the amount of NG.
Quote: Originally posted by Hennig Brand  |
An ice water bath is an excellent way to cool a nitroglycerine nitration reaction mixture.
Water heat of fusion: 334J/g (334kJ/kg)
Mass of ice needed for cooling per gram of glycerine nitrated assuming no heat transfer to surroundings:
(1.43kJ/1g Glycerine) * (1kg Ice / 334 kJ) = 0.0043 kg Ice/1g Glycerine Nitrated (4.3g Ice/g glycerine nitrated)
Ice needed for synthesis of 1L of NG = 1000mL(1.6g/mL)(4.3g Ice/g glycerine) = 6880g of Ice (6.88kg of Ice)
This is assuming no heat transfer from the surroundings! In reality the amount of ice required could be much more.
|
Here is the correction:
An ice water bath is an excellent way to cool a nitroglycerine nitration reaction mixture.
Water heat of fusion: 334J/g (334kJ/kg)
Mass of ice needed for cooling per gram of glycerine nitrated assuming no heat transfer to surroundings:
(1.43kJ/1g Glycerine) * (1kg Ice / 334 kJ) = 0.0043 kg Ice/1g Glycerine Nitrated (4.3g Ice/g glycerine nitrated)
Ice needed for synthesis of 1L of NG = 1000mL(1.6g/mL) / (227.09 g/mol) * (92.09382 g/mol) * (4.3g Ice/g glycerine) = 2790g of Ice (2.79kg of
Ice)
This is assuming no heat transfer from the surroundings! In reality the amount of ice required could be much more.
What a difference a little slip can make!
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Ive bought these of ebay they work a treat controlling heating elements and ac brushed motors. cheers nuxy.
http://www.ebay.com.au/itm/Adjustable-Voltage-Regulator-AC-S...
[Edited on 15-2-2015 by nux vomica]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Just my 50 cents as a side note!
Determination of organic alkanic halogens can be done with AgNO3 (in methanol or aceton...avoid ethanol at all costs because of the risk of formation
of sensitive Ag-O-N=C (silver fulminate)!)
-Reactivity goes in the way I > Br > Cl
-Primary halogen react faster
R-X + AgONO2 --> R-ONO2 + AgX
-Secondary halogen react at a slower rate or with mild heating
R2CH-X + AgONO2 --> R2CH-ONO2 + AgX
-Tertiary doesn't react
R3C-X + AgONO2 -//-> R3C-ONO2 + AgX
-AgX can be recyled by electrolysis or by photolysis into metalic Ag and X2; eventually by cementation upon contact with metallic copper.
-Chloride can be converted into bromide and iodide by reacting R-Cl with saturated NaBr or NaI aceton solutions (NaCl precipitates out).
-Polyhaloalcanes can be done OTC from polyols with concentrated HCl, ZnCl2 and heat.
From this it is obvious that glycol dinitrate (EGDN) and propantriol trinitrate (NG) can be made by simply mixing Cl-CH2-CH2-Cl, or ClCH2-CHCl-CH2Cl
aceton and slight excess of AgNO3...with NO HEAT NOR RUNNAWAY RISKS!
This technique must be applicable to higher nitrate esters like PETN from (C(CH2Cl)4), ETN from 1.2.3.4-tetrachlorobutane (chlorinated erythritol),
pentachloropentane (chlorinated xylitol), hexachlorohexane (chlorinated mannitol or sorbitol), .... and to make PVN from low molecular PVC.
The only limitation for most is the cost of AgNO3... but I personnaly own about a kg of that stuff and that is the reason why I thought about this.
Last non negligible aspect... if I-CH2-CH2-I is more suitable than Br-CH2-CH2-Br itself better than Cl-CH2-CH2-Cl, the prevalence of Iodide is limited
by its cost/availability over Bromide and Chloride and by the unstability of vicinal iodides...
1.2.3-triiodo-Propane easily turns into allyl iodide and iodine!
I-CH2-CHI-CH2-I ==> CH2=CH-CH2-I + I2
[Edited on 16-2-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Very interesting method you have laid out for us. I probably have a few hundred grams of silver nitrate, but I hang onto it for silver azide,
acetylide, fulminate, etc. Though interesting the amount of stages and expensive materials involved in the process including recycling is a lot in
comparison to the traditional approach using mixed acid and glycerine. I don't think that there is any need of a runaway even with up to a 1L NG batch
size as long as a few basics are understood (I apologize for the lack of care employed when going through the numbers the first time). I am kind of
looking forward to attempting a relatively large batch at his point now that things are quantified reasonably accurately with regards to heat.
[Edited on 16-2-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The other avantage of AgNO3 method is that no concentrated HNO3 or H2SO4 are needed.
Last but not least danger of scaling up the HNO3/H2SO4 method for nitrating polyols comes from:
-While the volumes increases, the relative cooling surface decreases
Just as an example consider a cubic reactor; the volume is full, but the active cooling happens by 5 sides, upside is left to open air (natural
cooling is considered ineffective).
Height= 1 cm
V= 1 cm³
Cooling surface= 5 cm²
S/V geometrical cooling efficiency= 5
Let us double the dimensions
Height= 2 cm
V= 8 cm³
Cooling surface= 20 cm²
S/V geometrical cooling efficiency= 2,5
One more doubling
Height= 4 cm
V= 64 cm³
Cooling surface= 80 cm²
S/V geometrical cooling efficiency= 1,25
And another doubling
Height= 8 cm
V= 512 cm³
Cooling surface= 320 cm²
S/V geometrical cooling efficiency= 0,625
In the industry such effect have had severe consequences and have resulted in most large chemical incidents. Scaling up from the lab-scale to
pre-pilot plant and full pilot plant must be studied in details. Even a simple doubling has lead to lethal explosions.
-Mixing becomes less efficient and hot spots can easier happens...
Because you cannot increase the speed of mixing by much...cavitation will occure and with those the risk of detonation of the formed NG increases.
-The rate of addition of reactants must be addapted to the scaling up the larger the batch; the slower the addition.
[Edited on 15-2-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I have been through a lot of the important heat transfer calculations in the last half a dozen posts or so. I don't mean to sound arrogant or
careless, but I think I understand the cooling and mixing requirements better than most now. You make a good point about mixing requirements, and I
think the idea would be to use a very large slow moving paddle or some other stirring attachment. Large and slow moving so that good mixing is
achieved but also very gently.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@Hennig Brand,
I don't put your experience on the line, but it needs to be pointed for less carefull users.
The main problem is that for effective mixing, one usually need a turbulent mixing and not a laminar one...
A slow, gentle mixing at microscale is in fact quite turbulent due to wall effects but at higher scales it will be close to laminar and will lead to
dead zones (unproper mixed zones) very prone to hot spots...
Shock sensitivity of NG precludes the use of faster mixing rate (rpm).
So while general macroscale thermic indicators might prove to be on the safe temperature side...locally hot spots will form with increased reaction
rate (reaction rate and heat generation is quadratic as a function of temperature) so hot spots will generate local runnaway that may very fast
propagate to the the full batch.
-->In the case of large NG batch because of the inherent sensitivity of NG to shock/heat... this calls for serious troubles.
In other polyols nitration, the mixing may also induce friction of the polyol polynitrate ester cristals.
Other aspects for mixing of explosive materials:
-In some cases mixing of non polar solvents may induce static electricity build up and high voltage sparks inside the fluid.
-If viscosity is high, mixing at high speed will convert kinetic energy into friction energy and transfer a good deal of heat to the fluid. I have
noticed this effect while mixing viscous solutions of silicone oils in ethanol with lab propeller blade at high speed.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I was planning an using a piece of Teflon sled runner, which would act like a large stir bar except that it would be driven from above by shaft and
not from below by magnetic coupling. I already have a powerful cordless drill and power supply and speed control for it, so that much is sorted out.
Did you have any suggestions about the type of mixer attachment that would be suitable? I would be pleased to hear any.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Hennig Brand  | | I was planning an using a piece of Teflon sled runner, which would act like a large stir bar except that it would be driven from above by shaft and
not from below by magnetic coupling. I already have a powerful cordless drill and power supply and speed control for it, so that much is sorted out.
Did you have any suggestions about the type of mixer attachment that would be suitable? I would be pleased to hear any. |
I would use propellers that would induce much trubulence at low speed.
Like multiple propeller blade on a single axis or cheese slices (with holes  with
different paterns and size to break laminarity on next coming mixing wall) with
different paterns and size to break laminarity on next coming mixing wall)
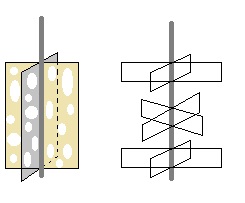
Or something more complex...like

But I think complexity is not needed here.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
..
20
21
22
23
24
..
27 |