| Pages:
1
..
3
4
5
6
7
..
9 |
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I thought some might be interested to know;
Pythagoras is mullite, it's a brand name Morgan Thermal Ceramics use for the refractory. MTC manufacture a huge percentage of the refractory products
in the UK, like SuperWool, ceramic papers, cements and so on.
Mullite;
| Quote: | | Mullite or porcelainite is a rare silicate mineral of post-clay genesis. It can form two stoichiometric forms 3Al2O32SiO2 or 2Al2O3 SiO2.
|
- From wiki
This is interesting, as others have mentioned how nichrome like wires can not come into contact with silica glass, but there is a (sometimes large)
percentage of silicates in the lower temperature refractories. I have also been on forums related to kiln building, wherein they are using nichrome or
kanthal inside Quartz tubes or Quartz as a former for the element. Duralite even sell a commercial element of this design.
Pythagoras;
| Quote: | Pythagoras
Most economical, Mullite material for kiln components.
Type 610 according to DIN VDE 0335
Application temperatures up to 1400 °C
Very good chemical resistance against gases free of fluorine
For kiln working under normal conditions Pythagoras has a good thermal shock resistance and good mechanical strength
Pythagoras is a very economical material being used as impervious protection sheaths and insulators for temperature measurement
|
- From Morgan Thermal Ceramics
The economical part will be of interest to a lot of people, as pure alumina tubes of a similar size are roughly five or ten times more expensive. As
Quartz is usable to only 1100C before mechanical problems start, there is a strong incentive to avoid the pure alumina materials if at all possible.
I decided to rebuild the hotbox of my furnace, as (on opening it) I discovered it was not in a nice state inside. I am now trying to contact multiple
people (I've already lost count) to investigate if it is a good idea in terms of element longevity to have them in direct contact with silicate
containing refractory. I'm waiting for a phone call back from the technical boys at Morgan for some more advice on silicates and direct contact.
Morgan actually have a factory about ten minutes drive from my house, so I've been trying to weedle a visit out of them thinking it might be
interesting to see the products being made.
I noticed GC and Magpie were having problems getting their cement to stick to the elements and discovered an interesting snippet from this site;
| Quote: | Electrical Elements
New elements have a greasy residue left from the wire manufacturing process, which may cause the ITC 213 to flake off if not removed. One method is to
pre-fire elements after stretching for 5 to 10 minutes to achieve at least cherry-red glow. If this is not possible, the elements can be heated in a
furnace or kiln at 700oF for 30 minutes. Allow to cool.
The ITC 213 can be applied as described above or can be applied by dipping. Using a wide shallow pan, empty the ITC 213 into the pan and add 1/3
water, mixing well. Dip the entire element except for the lead wire into the ITC 213 mixture. After dipping, shake the element to remove excess
coating and hand to dry for several hours or overnight. The elements are now ready to install. |
[Edited on 21-6-2011 by peach]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by peach  |
The economical part will be of interest to a lot of people, as pure alumina tubes of a similar size are roughly five or ten times more expensive. As
Quartz is usable to only 1100C before mechanical problems start, there is a strong incentive to avoid the pure alumina materials if at all possible.
|
This large price difference may be a function of where you live. On this side of the pond there wasn't that much difference in price between mullite
and alumina when I bought my alumina tube 3 years ago. It was $101 then.
Quote: Originally posted by peach  |
I noticed GC and Magpie were having problems getting their cement to stick to the elements and discovered an interesting snippet from this site;[/url]
Electrical Elements
New elements have a greasy residue left from the wire manufacturing process, which may cause the ITC 213 to flake off if not removed. One method is to
pre-fire elements after stretching for 5 to 10 minutes to achieve at least cherry-red glow. If this is not possible, the elements can be heated in a
furnace or kiln at 700oF for 30 minutes. Allow to cool.
The ITC 213 can be applied as described above or can be applied by dipping. Using a wide shallow pan, empty the ITC 213 into the pan and add 1/3
water, mixing well. Dip the entire element except for the lead wire into the ITC 213 mixture. After dipping, shake the element to remove excess
coating and hand to dry for several hours or overnight. The elements are now ready to install.
|
not_important pointed out this pre-oxidizing technique on p. 4 of this thread already.
I didn't use ITC-100 but ITC-213. ITC-100 may have been more appropriate, but my tube furnace continues to function well. It wasn't a problem of not
sticking to the kanthal wire but a problem of cracking. But since it did not spall off it seems to have been a non-issue.
For those in the US who would like to buy Kanthal wire by the foot, check the pottery suppliers.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
metalresearcher
National Hazard
   
Posts: 731
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
I got 30 meters of 1mm Kanthal A1 wire for $36 via ebay and rewired my furnace with it.
http://shop.ebay.com/i.html?_dlg=1&_jgr=1&LH_PrefLoc...
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I noticed the pre-oxidising mention earlier, but forgot whether or not it was in relation to the refractory flaking off.
$101 seems in roughly the right area for alumina. But here's a comparison on today's UK price difference between the two;
| Quote: |
RCA Tube 35 mm O/D X 28.8 mm I/D alumina X 1000 mm long at unit cost £36.00 (1 in stock)
Pythagoras tube 29 mm O/D X 23 mm X 760 mm long unit cost £5.50 (100 in stock)
|
Six times cheaper. These prices don't include VAT, which is currently 20% in the UK. 36 pounds becomes £43.20, which is about $70. Plus on the
delivery, $86 ish.
[Edited on 21-6-2011 by peach]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by chief  | Just to share 2 Ideas (not from me, but tricks of the trade) on reaching really high temoeratures:
==> up to 1700 [Celsius]: Mo-wire around Al2O3-ceramic-tube [also commercially available as furnaces]
==> up to 3000 [Celsius]: Wo-tube, current directly through the tube
The latter may be adapted,using cheaper stainless-steel-tubes (limited lifetime, but throw-away available from hardware-stores), which should hold up
to 1300 [Celsius] too; then it would be only a matter of contacting them, wrapping some isolating-mat (even maybe rockwool) around them. Since the
resistance rises with temperature the electrical power would be dissipated within the isolated zone, contacting therefor should be easy enough (if
necessary through welding).
As power-supply: One thick additional secondary winding around the welding-transformer, eg. . Within such stainless-steel-tubes then, at lower temp.,
even pressure-experimentation could be done (catalysts in the tube, have fun making HNO3 etc.), since in the hardware-store there are also other
stainless-steel-components available,usually to make waterpipe-connections etc. .
Maybe someone could succeed in Fischer-Tropsch-gasolining from coal-water (50 Bar, 200 [Celsius]) ?
[Edited on 8-10-2008 by chief]
[Edited on 8-10-2008 by chief] |
Interesting note there on the tungsten.
Remember that all normal grades of stainless have service temperatures below 1000C. I would be grateful if they didn't start slowly sagging at that
point. Pressurising them could get nasty.
Silicon carbide and moly D elements are available as tubular forms that'll manage 1400 to 1850C, but they are expensive.
I was having a click around on the Kanthal site and spotted this.
"Kanthal furnace tubes can be used at temperatures up to 1250°C (2280°F) and are available in sizes from 26 to 260 mm (1.02-10.2 inch) outside
diameter."
Wonder how much they are.
One of the good things about the silicon carbide and moly D tubes, that these lack, is that they feature spiral sections for the hot zones, so the
ends where they touch the refractory and wiring remain a bit cooler.
|
|
|
metalresearcher
National Hazard
   
Posts: 731
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
W and Mo wires are NOT a good idea as I read earlier on this forum. Both metals oxidize quickly at those temperatures unless you have a proper vacuum
or inert gas (Ar) furnace chamber.
There are also coiled MoSi2 elements up till 1900°C available from www.mhi-inc.com.
Another option is using a propane burner with forced air (using a $10 air mattress inflator suffiices). This heats to 1500oC.
Her my video:
<iframe sandbox width="425" height="349" src="http://www.youtube.com/embed/Z2wGV6g7I6E" frameborder="0" allowfullscreen></iframe>
[Edited on 2011-6-22 by metalresearcher]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Thanks for the video MR!
Graphite is used in commercial furnaces that need to reach 3000C and where a direct arc, plasma or flame aren't acceptable.
That is a ridiculous temperature for the majority of what people on here will want to do, and expensive to cater for (it'll boil aluminium oxide and
melt magnesium oxide & zirconium dioxide  ). ).
<object width="560" height="349"><param name="movie"
value="http://www.youtube.com/v/DWrisy1_8_U?version=3&hl=en_GB"></param><param name="allowFullScreen"
value="true"></param><param name="allowscriptaccess" value="always"></param><embed
src="http://www.youtube.com/v/DWrisy1_8_U?version=3&hl=en_GB" type="application/x-shockwave-flash" width="560" height="349"
allowscriptaccess="always" allowfullscreen="true"></embed></object>
[Edited on 22-6-2011 by peach]
|
|
|
metalresearcher
National Hazard
   
Posts: 731
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
@peach:
I performed experiements with the electric arc including melting MgO and boiling metals.
www.metallab.net/arcmelt.php
The graphite heating is used in graphitizing coke with the Acheson furnace, heating coke pressed in rods to 3000oC.
[Edited on 2011-6-23 by metalresearcher]
|
|
|
Thor
Harmless

Posts: 19
Registered: 13-6-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by peach  |
| Quote: |
RCA Tube 35 mm O/D X 28.8 mm I/D alumina X 1000 mm long at unit cost £36.00 (1 in stock)
Pythagoras tube 29 mm O/D X 23 mm X 760 mm long unit cost £5.50 (100 in stock)
|
Six times cheaper. These prices don't include VAT, which is currently 20% in the UK. 36 pounds becomes £43.20, which is about $70. Plus on the
delivery, $86 ish.
[Edited on 21-6-2011 by peach] |
Could you tell me the source of these prices? Have tried a few suppliers and the price's seem to differ hugely.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Are you in the UK? Because shipping would probably make the difference between your local prices and mine not all that much if you're in the US.
http://www.almath.co.uk/ has it, but only in specific sizes.
I bought mine from Anderman, who seem to have more than one site. The one I initially contacted was something like 'Technical Ceramics'. Here's
another with the name Anderman in their emails. http://www.earthwaterfire.com/
I was pissed off to discover the postage was going to be £15. The tube it's self was about £10 I think, and just over a foot long. So it would have
gone in one of those poster tubes and the royal mail could have sent it for about a quid.
I've since tried Garage Chemists nichrome wrap method as well, using this tube, and it works a charm.
Keep in mine that cutting this stuff yourself is extremely difficult. It's so hard HSS hacksaw blades don't even scratch it, they just leave a shiny
mark where the blade has worn down. I tried using a diamond disc in a tile cutter, but it continually shattered a scrap piece I was using. Get them to
cut if it needs chopping down, they have special high speed diamond saws with precise guides to hold it in place as it goes.
{edit}Do any of the US guys know of any companies who still do by ground international shipping? USPS seems to have dropped that 4-6 week service, so
I keep getting insane 'express by air' quotes on stuff to the UK. Some of them aren't far off the amount it'd cost for me to get on a plane, fly
across the ocean for seven hours and collect things in person. It's funny, imagining things I've bought sitting on a seat in the plane on their own,
being served drinks with their safety belt on.
[Edited on 25-7-2011 by peach]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I have a method for cutting pythagoras tubes at home.
It involves two persons, one is holding a powerful angle grinder with a disc for cutting concrete, grinder sitting on the ground and disc facing
upwards, and the other one holds the tube to the spinning disc while constantly rotating the tube, slowly cutting deeper with each rotation. There is
a little chipping at the cut, but after cleaning up the cuts with a diamond grinding bit only about 1cm of tube is lost.
I agree that you should always let the supplier do the cutting if possible as this method is obviously somewhat dangerous both for the tube and for
the workers, though I've made several cuts this way and never broke a tube.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by garage chemist  | I have a method for cutting pythagoras tubes at home.
It involves two persons, one is holding a powerful angle grinder with a disc for cutting concrete, grinder sitting on the ground and disc facing
upwards, and the other one holds the tube to the spinning disc while constantly rotating the tube, slowly cutting deeper with each rotation. There is
a little chipping at the cut, but after cleaning up the cuts with a diamond grinding bit only about 1cm of tube is lost.
I agree that you should always let the supplier do the cutting if possible as this method is obviously somewhat dangerous both for the tube and for
the workers, though I've made several cuts this way and never broke a tube. |
That's pretty much what I tried with the RCA.
I managed to make a good, clean groove in it, but it was so easy for it to chip back an inch or so. I tried on some scrap a few times, but could never
get a nice, clean edge on the diamond tile saw.
I think one of those diamond blades Dremel sell might have a better chance, but I'd still go with, "get them to do it". Most of them will do it 50p or
free, saving you the possibility of loosing pounds on mullite or tens of pounds on alumina.
[Edited on 25-7-2011 by peach]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by peach  | It's funny, imagining things I've bought sitting on a seat in the plane on their own, being served drinks with their safety belt on.
|
 That's damn funny, peach. That's damn funny, peach.
I've cut the tops off assay crucibles (mullite?) using a masonry/concrete blade in my table saw. This gave a fairly clean cut.
[Edited on 25-7-2011 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|

| Quote: | | It wasn't a problem of not sticking to the kanthal wire but a problem of cracking. |
I have also noticed cracking, despite pre-oxidising the wire and leaving the cemented result overnight and then in the oven all day, gradually
increasing the temperature from 70C to 250C to cure the cement. Solution? Bake and then re-cement with a thinner layer? Must be water loss and
shrinkage.
This reminds me of rendering the garden. A week of mixing half a ton of render by hand, troweling it on by hand in the hottest summer on record, stuff
cracks in the lower parts my brother did; lack of PVA / SBR / admix? Render is a magical thing.
The tubes that cracked for me on diamond cutting were pure alumina. Maybe Mullite is less prone to it. I didn't give it a try. I have some iron ladle
bricks and damn......! They're tough!
[Edited on 25-7-2011 by peach]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I've never "rendered" anything (had to look up the meaning as this seems to be one of those many UK terms not generally used in the US). I have done
some concrete work occasionally, though. Along with getting the right water:cement ratio it is important to keep the concrete wet until it has
developed sufficient strength through hydration. I usually covered it with plastic film, or cotton towels kept wet, for about 7 days. Have never had
any cracking, but this was bulk material for foundations, not thin layers.
I don't know if any of this would apply to the commercial product I put on my tube furnace, however. I just followed directions, which did not
include keeping it wet, IIRC.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Thor
Harmless

Posts: 19
Registered: 13-6-2011
Member Is Offline
Mood: No Mood
|
|
Peach: I'm in the UK, so these companies are ideal. Anderman is one that I have contacted, no reply yet though. Is there any particular size of tube,
my hood is quite small, so if i can use a smaller length of tube then that would be better suited. Also will need to try and source a quartz tube with
ground glass joints too, which never seems to come up on ebay UK.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Nah, you're not getting a decent formed, taper quartz tube with caps via eBay any time soon! 
The average 'home chemist' is far more interested in making kEwL thermite than doing anything involving quartz.
My mullite tube is about 29mm ID I think, and it's 460mm long. They usually come as 760 or a metre. Most companies only do things by the stick or
sheet; even though they'll chop it up, you have to pay for the whole length.
I am about to order a quartz liner myself and the guy who's making it is doing it for a reasonable price. If you can decided what size you want yours
to be within a week or two, I may be able to get him to do it cheaper by adding more to the order. He also has quartz wool, can make the custom tubing
adaptors, boats and all that stuff to go with it. I think mine is around a hundred pounds for the tube, caps and some wool.
As far as Anderman go, I think you'd be better firing up your fingers on the phone's keypad. The guy I spoke to also gave me the wrong email address
over the phone.
I've had the vacuum people on the phone three times now, to repeat my card number. My last experience with another vacuum company was the same, and
then I got a letter from my bank telling me they wanted money I'd already given them. Some companies have not joined the high tech era of the pen and
paper note. 
[Edited on 26-7-2011 by peach]
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
Bump.
I have not read the thread completely.
Has anyone tried using those quartz tubes in domestic heaters as elements in a furnace. The tubes have a coil of Nichrome (I presume) in them.
I am going to construct a furnace. Not very high temperature, perhas up to 1100 C.
Are these quartz tubes resistent to hot water vapour? I am not putting the hot tubes in contact with cold steam or water BTW. The tubes in question
are not clear as they contain little air bubbles, I believe.
I would love to purchase a Moly Sci element to play with.
http://www.duralite.com/store/scripts/prodList.asp?idCategor...
The price is a bit hot too!!
[Edited on 30-5-2013 by jock88]
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Furnace up to 1200-1300C
I need to make a furnace that can reach temps up to 1300C MAX. It could run on electricity, but I would like having it with propane burner, wherever
this could be possible to make. I need it for making calcium oxide from calcium carbonate and calcinating and other processes too.
There is furnace on this thread mentioned:
https://www.sciencemadness.org/whisper/viewthread.php?tid=14...
What materials could the reactor be made of to hold up for this? I have no zirconia and other stuff available, so common structural materials,
fireclay, fire mortar (cement used to make fireplaces), common clay and this kind of stuff are to go.
I can have resistance wire, but could I use 6kW propane burner to reach temps up to 1300C? The volume I'd like to have for the pot would be 3-5
liters.
Could stainless steel (MP 1510C) used for max. 1300C processes? It would be easiest to put a pot of stainless into fireclay, wrap resistance wire
around it and make a cover for it.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
The Kiln Book, by Frederick L. Olsen.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
How much temperature would an 2kW induction heater generate, would it reach 1000C without trouble if I put one under the oven and insulate it with
double ceramic layer?
I think SS crucible would be enough to hold CaO and other processes which limit to 1000C.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I don't think he gets it, <strong>watson.fawkes</strong>.
<strong>testimento</strong>, what we was undoubtedly implying is that your questions are more complex than you realize, and cannot be
simply answered. You'll need to do some further research, perhaps studying the book he has recommended.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Yeah, I understand Im looking a simple solution for complex task. I dont need pharmaceutical performance though, I just need reduce some calciums and
other stuff which should go in quite harsh conditions, compare to mining industry.
I have following design in mind (ATTACHMENT)
a steel cooking pot in
cover it with clay of 1-2cm thick
put charcoal in the mantle
add another layer of clay of 0.5-1cm thick
Cover the lid with same structure
Would this reach 1000-1200C temp with LPG gas burner? Should the bottom be covered with clay layer too, would this hinder the delivery of energy into
the container?
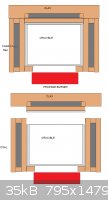
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
dont remember which thread it was but I remember reading a comment by one of the sci mad members saying stainless oxidizes above ~600/650'C. I lucked
out and got a nice big quartz tube 147mm x 152mm optical grade for 11$ + 11.95$ s&h off ebay. so <25$ was a great purchase. what you going to
use to monitor temp, control temp? did you calculate how much wire and all that you are gonna need( if you go electrical)? you don't wanna pull so
many amps you blow a breaker every time it turns on. it's a big project to get there and not a lot is easy about it. I am in the process of building
mine, in scant spare time, but slowly it is happening.
instead of charcoal liner you could try rockwool. I haven't done the heated tests yet but they sell cubes for transplanting seedlings and clones in a
garden supply shop. they come all stuck together in small rectangles that are easily cut. might make a better insulator, might melt to a pile of
goo, don't know yet.
http://en.wikipedia.org/wiki/Rockwool
the wiki page says 700-850'C so may not be great for you unless they are shielded a bit from the core temp. but something to think about.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Yes I considered rockwool and glass wool and other wools, but they do have limited temps of 400-800C, and some few ceramic special wools can hold up
to 1300C. Asbestos was another one, but that is obviously a rather too nasty one for this purpose.
The stainless will form an oxide layer at high temps but as long as no mechanical forces are applied, it just sits there, as far as I have tested. The
surface layer flakes off when you cool the pot, so in the end after a couple of hundred runs, the reactor bottom may just fall off. I believe I will
not be conducting more than a few dozen runs with the reactor until it goes to dump yard so I dont concern about that a lot.
I use triacs with potentiometers to control the power of the heaters. I have controllers that limit max power to 2000, 3000 and 3800 wats,
respectively, so it will just sit there at the designated temp. I have one with my 2kW chlor-alkali cell too and it works like a clock.
I'll give a try for this reactor type and see if I can get it hot enough. The propane should give 2000C temp at the flame core and melting lead with
it is a minute job, but I made a test today where I used CaCO3 in a steel pot with double radiation shieldings and the temperature went so high the
plastic knob on top of the lid of the pot self-ignited and charred into carbon so the temp was at least 500-600C, but IDK will it reach 1000C. If it
wont, I will consult my electric company via nichrome.
[Edited on 4-7-2013 by testimento]
|
|
|
| Pages:
1
..
3
4
5
6
7
..
9 |