stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
the proper use of heating tape on a vigreux type column...
please help me on this one:
i want to fracionally distill an organic compound, yet i have no idea what temperature to choose for the heating tape wound around the column. right
at the compounds estimated bp.?
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Using heating tape has the same purpose as isolation of column.
Do you really have/want to use this tape ? What is bp ?
Слава Україні !
Героям слава !
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I'd like some discussion on this too. I was discouraged, here, from using one. I undertsnad the idea is to maintain the column at the temperature of
the vapour rising from the still pot. I was told a shitty separation was from runing the column too hot. However a column with an evacuated,
silvered jacket would accomplish the same thing as having a heating tape or wire at the temperature of the vapour wouldn't it?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
The #1 killer of efficiancy on fractional distillations is the use of a hot plate instead of a mantle.. there is absolutely no way for the column to
equalize worth a shit with the heat constantly cycling on and off.
Also, vigreaux columns have a much much lower height equivalent to theoretical plate ratio vs a properly packed hempel column.
I've pretty much come to the conclusion that in most instances heating tapes are unecessary and all that is needed is some decent pipe foam
insulation... in fact, some cooling is actually beneficial as that helps to generate reflux.
And you cannot run a column too hot, only too fast. Faster you go, the lower the efficiancy. Only way to counter that is to lower the heat or increase
the width and length of the column.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
i'm limited to using my 60cm vigreux column (lacking the funds to buy a packed column, which also has much more holdup - i'm distilling 50-100ml
quantities).
the compound has an approximate bp of 260°C@760mmHg. i'm distilling it in vacuo so it comes over at around 115°C. there is a lot of heat loss in the
column, since it only has a simple glass jacket fitted with two rubber o-rings - hence the use of heating tape.
i have a lot of glass wool which would make some nice insulation, yet i'm trying not to work with glass wool due to the fiber dust being quite some
health hazard.
evil_lurker, you can in fact run a distillation "too hot" (not a column in particular) - i have had one incident where i cranked the heat up too much,
to the point where the covalent bonding energy in the compound was lower than the heat of vaporisation. this ended up with distilling over a copious
amount of very, very expensive tar 
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
There is an interesting discussion on the use and modification in using this type of distilling column ............solo
http://www.sciencemadness.org/library/books/Research_Techniq... -
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
thanks, solo! this really is a useful document. the grignard soxhlet method on page 55 rocks, especially if you want to prepare e.g. BenzylMgBr.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
So, what if you have a very expensive, well made hotplate that creates very stable temps?
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
There is no such hotplate as far as I'm aware. Hotplates have an on/off regulation, hence there is no way for them to maintain a stabile temperature
of the plate. The best you can have is a hotplate magnetic stirrer with an oil bath equipped with an external thermostat sensor. These are however
much more expensive than the simple ones without an external sensor, and they still run on an on/off regulation resulting in an deltaT of about 10K
(though depending on the model). This is still a much higher deltaT that can be achieved by simply using a triac rheostat or other continuous current
regulation like the ones on the heating mantles.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
| Quote: | Originally posted by Nicodem
There is no such hotplate as far as I'm aware. Hotplates have an on/off regulation, hence there is no way for them to maintain a stabile temperature
of the plate. The best you can have is a hotplate magnetic stirrer with an oil bath equipped with an external thermostat sensor. These are however
much more expensive than the simple ones without an external sensor, and they still run on an on/off regulation resulting in an deltaT of about 10K
(though depending on the model). This is still a much higher deltaT that can be achieved by simply using a triac rheostat or other continuous current
regulation like the ones on the heating mantles. |
Think of it like this... distillation removes heat. By the time hot plate's thermostat realizes that the temperature has cooled down due to all the
oil, glass, and metal that interferes with efficiant heat transfer, the distillation rate has slowed.
As the hot plate thermostat cycles back on, by the time the flask gets the heat the hot plate has thrown more heat than necessary to bring the flask
back to temperature, resulting in a "surge" effect.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | Originally posted by evil_lurker
As the hot plate thermostat cycles back on, by the time the flask gets the heat the hot plate has thrown more heat than necessary to bring the flask
back to temperature, resulting in a "surge" effect. |
i have been using a hot plate with oil bath for this distillation, which has a socket for a thermometer that you can dip in the bath and have your
temp regulation this way. yet i havent checked how much the "overshoot" was when the hot plate cycled on heat.
i have also used a heating mantle (PILZ brand), hooked up to a regulating device which modulates power by switching the current on and off at defined
intervals, corresponding to the 1-10 dial setting on the device. i had to use this since the 3 level switch on the mantle delivered either too much or
too little heat.
i guess either setup is faulted with temp fluctuation.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Get on Ebay and buy a Glas-Col Series O heating mantle and controller... you can usually find them in good shape quite cheap ($30-75).
Hell, this last week I got a 12L TM series mantle brand new for $150... they normall list for around $550-600.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I don't know how hot plates crept in to the discussion, I must have missed the beginning sentence. I do believe that an oil bath heated by a hot
plate will work out to being a better heat source, thpugh messy and inconvenient, than a mantle. Reasons: the oil can hold a nearly constant temp
while the plate cycles .. buffering the temperature, so to speak, by way of its heat capacity. The oil bath provides nearly uniform heat to the
flask whereas the mantle will have hotter and cooler zones, nearer or away from the heating element. I had a chem prof who believed so sincerely in
them we were required to use oil baths in ug organic classes and he tried to convince us to use them later on. He had a killer Podbielnak column he
made from tubing and cork.
I wanted an opinion on Snyder columns while we're talking efficiency. Seems like a bubble plate column in essence. How much separation per cell?
cell = plate?? This is P Chem or ChemE or something equally obscure to me.
Solo's reference, cited above, offers these observations on distillation columns.
"Since any effective distillation requires that a close to true equilibrium
exists between the boiling liquid and condensing vapors, it is important to
minimize external heat losses in the system. This is most easily accomplished
by enclosing the fractionating tube in a vacuum jacket and placing the
jacketed tube in a second jacket that can be heated electrically. After the
liquid is refluxing steadily in the column, the external jacket temperature
should be adjusted to about 1-2° below the reflux temperature. If the column
floods at the bottom, either the boil-up rate is too great or the column jacket
is too cold. If the column floods at the top, the column jacket is probably
too hot. Another common source of flooding with packed columns is the
presence of a constriction due either to improper packing or to a rearrangement
of packing caused by bumping or a prior flooding condition."
[Edited on 8-1-2008 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
It is my view that the first best choice for any fractionation column is a vacuum jacketed and silvered one, regardless of the type of column (Hempel,
Oldershaw, Snyder, Vigreaux).
This removes all variations due to good/bad technique of lagging. Therefore, the operator's attention can be focused on the other vital parameters of
the still design and use. These include
Proper matching of heat input to condenser and cooling capacity
Proper pot size for column length and diameter (and that varies with type)
Reflux ratio
Boil-up rate
And time to allow system equilibration at 100% reflux before gingerly reducing the RR and starting to take off lowest boiling fraction.
I would think that even a cursory review of the theory and practice of fractionation would clearly indicate that if an external heat input is to be
made to the column, in lieu of normal cacuum jacketing or insulation lagging, the temperature of the heating tape or cord needs to be less than or
equal to the LOWEST BOILING compound in the mixture, as determined by analysis or preliminary testing. Preferably, lower than that. Otherwise, there
cannot possibly be any equilibration. The still head will be overloaded with vapor. The desired liquid-vapor continuum cannot be achieved in the
column and thus, seperation will suffer.
So I would run a tape or cord at 10-20 C less than the bp of the lowest boiling component. But I would still rather do without the tape and either use
a vac.jacketed column properly packed, or else a well lagged unsilverered column.
The use of a heating tape makes sense if your mixture is high boiling even under reduced pressure and your are operating in a draft, particularly if a
silvered vac.jacketed column of suitable dimensions is unavailable or prohibitively costly - as they often are. I have a 1 meter, jacketed silvered
Hempel of 29mm ID that I bought on LabX for $70, but a new column like this would cost me several thousands of dollars. Oldershaw columns are even
worse cost wise.
I'd be inclined to spand a little money on a upper spherical mantle or a safety shield/poncho, any of which will prevent excess reflux from the upper
part of the pot. Inexperienced operators often just compensate by upping the mantle temp, to their regret - you ALWAYS want the MINIMUM heat input
that gets the mix to the top of the column eventually under 100% reflux, and good lagging.
I hope these remarks are helpful. Entire books have been written on this subject and at least one excellent one has been posted on this forum
previously, UTFSE. Doing fractionation without studying it is like trying to do brain surgery by reading a Classic comic book.
Sic gorgeamus a los subjectatus nunc.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
BTW:
Heating tape (cord or so...) does not set any temperature. "Temperature of column" changes continuously, in batch processes, during distillation -
such warming supplies only heat, losing via natural convection (mainly). It is obvious. The longer column, heat loses are the bigger but these loses
cannot be compensated by increased warming of RBF ( because lower part of column changes into steam cone - length of working part of columd
decreases). Of course, losig heat is sine qua non of practical fractional distilltion but in the case of too long column, vapors "get stuck"
in the middle of it and heating/insulating is needed.
Слава Україні !
Героям слава !
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
One of my goals is to gain the ability to perform difficult separations via fractional distillation. Several times I have needed that ability for
alcohols and esters with bps, say, 5-10°C apart.
One of the most important contributors to success is having a sufficiently high reflux ratio. At present I do not have a distillation head that would
provide control of this ratio. HS Martin makes such equipment but it is horribly expensive. The image below shows one such example:
Perhaps with a clever arrangement one could jury rig a still head along the same lines using condensers, stopcocks, and such.
Has anyone else been thinking along these lines? What are your thoughts? What have been your successes?
[Edited on 11-7-2013 by Magpie]
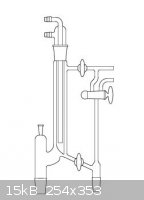
[Edited on 11-7-2013 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Have you considered a <a href="http://en.wikipedia.org/wiki/Spinning_band_distillation" target="_blank">spinning band distillation</a>
<img src="../scipics/_wiki.png" />? (Sorry, I don't have anything at the moment that can open a .doc file.)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I'm actually open to any kind of equipment that will allow difficult fractional distillations. I know that spinning band is probably the ultimate for
column design. The design with the little ball floats (Snyder) is also quite good, I understand. Right now I'm just concentrating on trying to get
good control over the reflux ratio as I know that it can be of prime importance.
Sauron has written profusely on this subject and seems to speak from experience. Some of this is upthread. Some is found in other threads.
Don't you have Word?
[Edited on 11-7-2013 by Magpie]
[Edited on 11-7-2013 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Well, it's customary to provide an image as an image file, not a Word document. 
Also, no, I don't have Word. This laptop came with Windows 8 (GAH!), and I haven't downloaded OpenOffice (yuck) yet.
|
|
|
adamsium
Hazard to Others
  
Posts: 180
Registered: 9-4-2012
Location: \ƚooɿ\
Member Is Offline
Mood: uprooting
|
|
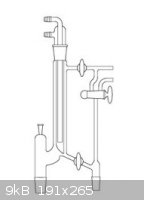
Here is the image, bfesser (and others):
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, thank you adamsium. I will go back and edit my original post. It seems I'm still learning about these things.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | | Right now I'm just concentrating on trying to get good control over the reflux ratio as I know that it can be of prime importance.
|
Yes. The separation ratio in the column is different issue than the reflux ratio accomplished in the still
head.
There's a good treatment of this in the book The Compleat Distiller, which I mention specifically because it's targeted at people building
their own gear (because they focus on alcohol production). The mention three ways of doing this: management by vapor, liquid, or cooling. Vapor and
liquid management mechanically divide a fluid flow into two flows. Cooling management splits the incoming vapor flow into a fluid flow (refluxed) and
a vapor flow (passed on an condensed). All this is tantamount to saying that you can build such a device before, within, or after the reflux
condenser.
|
|
|