| Pages:
1
2 |
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Methylation of phenolic aldehydes with MeI in DMF: 2-methoxybenzaldehyde
Methylation of salicylaldehyde by MeI in DMF
The Williamson alkylation of phenols by alkyl halides is a broadly reviewed reaction, with vast applications in the synthesis of key
intermediates. The alkylation of phenolic aldehydes can be a little trickier as these substrates are more sensitive and deactivated than phenols.
The methylation of a phenolic aldehyde, 2-hydroxybenzaldehdye or salicylaldehdye was carried out in DMF with methyl iodide in presence of KOH.
WARNING! : Methyl Iodide is a very toxic, cancerigen chemical, which is very volatil and thus easily inhaled. Maxium precautions
should be taken when handling such a reagent!
Procedure:
A 100mL single-neck schlenk, connected to the argon line and equipped with a efficient condenser, magnetic stirring, and vacuum inlet
was charged with 3.63g (55.00 mmol) 85% KOH and 30mL dry DMF [1], and quickly purged. Stirring was started, but the KOH
pellets didn't seem to dissolve. Heating to 70°C with an oil bath for 10min was fruitless [2] (Picture 1).
6.37g (52.60 mmol) of distilled 2-hydroxybenzaldehyde [3] (Picture 2)were diluted in 10mL
DMF in a 25mL schlenk, the solution was briefly purged, the solution added to the cooled, stirred KOH/DMF via a septum attached on top of the
condenser, under a flow of argon. The initially colorless solution took a yellow/green color after the first drops, and then gradually darkened to a
dark golden orange [4] (Picture 3). The KOH completely dissolved in 10 minutes at 25°C, then a bright orange solid
precipitated (phenolate) giving a dense suspension.
With maximum precautions (lab coat, goggles, 2 pairs of gloves, gas mask, efficient ventilation and dilute ammonia at hand), the
bottle of methyl iodide (Picture 4)was removed from it's protective bag, the parafilm removed, and 5mL (11.40g; 80.32 mmol)
of MeI were slowly redrawn with a 10mL glass syringe, and slowly left to dribble down the condenser through the septum, once the flow of argon had
been cut. The schlenk had been previously cooled to 15°c to avoid excessive vapors. The syringe was then rinsed with 2x5mL fresh DMF that were used
to rinse the inside of the condenser, then thoroughly washed with dilute ammonia, water, and acetone. The MeI bottle was resealed with parafilm, and
replaced inside its bag. The septum was removed from the top of the condenser, thoroughly washed with dilute ammonia, and replaced by a CaCl2 guard
[5].
The flask was left to stir for 1H30 at 20°C [6], turning to a lemonade-yellow suspension of a fine white salt (KI)
(Picture 5). The flask was then heated to 55-60°c on the oil bath; no reflux was visible on the sides of the schlenk or the bottom
of the condenser. Heating was continued for 12H, giving a dark orange suspension, which decanted relatively quickly when stirring was stopped, to a
dark red solution (Picture 6).
The flask was left to cool down for 2 more hours with stirring. A small sample was redrawn with a pipette with all necessary precautions,
diluted with water, a few drops of dilute NH3 added, and the vial shaken. Some white crystals formed as well as a dark orange oil at the bottom. A
little DCM is added, giving a light green organic extract, very similar to the color of the starting aldehyde. A few drops of 30% H2SO4 were added
until acidic, the aq. removed, and the DCM extract washed with 2 times its volume of dH2O. The organic layer turned dark orange; it is separated,
dried over a little amount of MgSO4 (the color reverts to light green) [7], and analyzed by TLC (DCM, 254nm silica plates), compared
to a sample of the starting compound. The plate was a little unclear, as the sample presented a large stain, a bit lower than the starting compound,
but nevertheless to close to confirm absence of the substrate[8], as well as a very small stain above. So another 1.25mL (2.85g;
20.08 mmol) of MeI were added with necessary precautions, and the schlenk heated to 60°C for 4h, a 1% dilute ammonia wash bottle connected to
the top of the condenser. The reaction medium turned to a darker red color. After 4h, TLC indicated completion of the reaction, with still a little
stain above the product.
150mL of a 10% NaOH solution [9] was prepared in a 300mL large neck erlenmeyer, and the cooled reaction mixture was slowly poured
in with steady stirring. A gas mask was used. A small amount of white crystals floated on the surface, and a yellow oil settled at the bottom, turned
to a semi-solid viscous goo after a few minutes (Picture 7).
25mL DCM were added, and the organic extract separated. In the separation funnel, a white solid precipitated, insoluble in water as well as DCM
[10]. The aq. and the white solid were transferred to a large beaker, carefully acidified with 15% H2SO4, and extracted with 3x25mL
of DCM, which totally discolored the aq layer.
The separated oil (the first extract) was equally acidified with 10% H2SO4, the organics combined, washed with 5% H2SO4, water, and 5% NaOH. The
first basic wash turned out light green and removed most of the brown/dark orange color of the organics. The second wash came out totally clear. The
organics were washed with 3 times equal volume of water, and dried over Na2SO4. TLC indicates a very small amount of a highly eluted impurity.
The basic extracts were combined, acidified with 10% H2SO4, and extracted with 3x10mL DCM. The extracts turned out cherry red. They were washed
with water, and dried. TLC showed a stain that corresponds to the stain found in the samples of the reaction.
The neutral extract was then transferred to a 250mL RBF, and the solvent removed on a water bath at atmospheric pressure, leaving an orange oil.
(Picture 8)Vacuum was applied to remove most of the solvent remains, and the flask cooled down under 30°C, at which point the oil
spontaneously crystallized (Picture 9). The oily solids were removed from the RBF, and weighed 7,42g after 24h in a CaCl2
dessicator.
The now crunchy crystalline mass (Picture 10) was transferred to 75mL beaker, and covered with 25mL of petroleum ether. After
slight warming (~30°C), DCM is added dropwise until total dissolution of the solids is attained (3mL). 5mL of pet. ether is then very slowly added on
the sides of the beaker. After 5min, crystallization starts, forming beautiful needles (Picture 11). After 30min, the sealed beaker
is transferred to the fridge, then to the freezer, and vacuum filtered. The crystals are washed with ice-cold pet. ether, and dried by suction on the
buchner (Picture 12). After air drying for 24h, the crystals weighed 5.26g (38.64 mmol), giving a 73.46%
yield. Single spot by TLC. (Picture 13)
Conclusion:A reasonable yield of o-anisaldehyde was obtained by methylation of salicylaldehdye by MeI in
DMF in presence of KOH. A large excess of MeI is needed, as excess KOH quickly destroys the alkyl halide. K2CO3 could be more adapted for such use.
Considering the toxicity of the alkylating agent, the long reactions time and the price, dimethylsulfate seems to be a much more practical reagent,
requiring much shorter recation times, being much less volatil though as toxic as MeI (or even worse), and being available by vacuum distillation of
sulfuric acic and methanol (albeit in low yields). When the bottle of MeI will be emptied, a reaction with Me2SO4 might be tried with a random
substrate. Results will be reported in this thread.
Argon atmospher was used to prevent oxidation of aldehydes during the long heating periods. Although very helpfull, it is not required for the
reaction to proceed well.
Notes:
[1] The DMF was used as is from a new VMR bottle. It was handled via glass syringe.
[2] Unlike what has been often mentioned, KOH doesn't seem to be soluble in DMF -or at a very small extent. The use of finely
powdered KOH could have helped dissolution, but grinding the pellets would have caused a lot of water absorption.
[3] The salicylaldehyde was synthesized by formylation of phenol using the paraformaldehyde/magnesium methoxide in toluene system in
very satisfying yields, see US5260487. Even after vacuum fractionation, the aldehyde had a green color (reagent grade is colorless) and it's
characteristic unpleasant odor. Single spot by TLC.
[4] Exactly the same color was seen at the end of the formylation, before acidification of the phenolate.
[5] A wash bottle would have been more adequate. There was a little concern about whether or not NH3 vapors could diffuse into the
schlenk and destroy some alkyl halide. The concern was rejected later on, and a wash bottle with ~2% NH3 was connected to the top of the condenser.
Health concern was predominant.
[6] To avoid having leaks of MeI vapors, the flask was maintained at RT hoping most of alkyl halide would react and not crawl up the
condenser during heating. Even though an efficient ice-cooled condenser was used, previous experiments with MeI showed that a certain amount of vapors
do climb up when refluxing. Not using any vapor trap inclined me to be precautious.
[7] This was pretty strange. The green color disappeared only during the water wash, not the acid, and comes back when the organic
layer is dried. This happened with all the samples. What happened?!
[8] A different eluant could have separated the stains more clearly. 10% DCM in hexanes will be considered in the future.
[9] This was a stupid mistake. The aq. base obviously hydrolyzed the DMF, producing a very strong dimethylamine smell; hopefully the
gas mask worked well. The smell stayed a long time though. The idea was to neutralize the excess alkyl halide without forming any imine with the
product, so ammonia could be used. Distilled water must be used for a proper workup.
[10] This solid was very intriguing. I have no idea of what it could be. A kind of adduct between the benzaldehyde and the
dimethylamine?
Pictures:
Picture 1: The KOH pellets in DMF.

Picture 2: Distilled salicylaldehyde.

Picture 3 : After addtion of the phenolic aldehyde.

Picture 4 : The Deadly Methylating Agent (brrr...)

Picture 5 : Reaction flask 10min after addtion of the alkyl halide.

Picture 6 : Reaction flask after 15h.

Picture 7 : Hydrolysis of the reaction mixture.

Picture 8 : Residual oil.

Picture 9 : After crysatllization of the oil.

Picture 10 : The crude product.

Picture 11 : The formed crystals.

Picture 12 : Filteration of the crystals.

Picture 13: Purified 2-methoxybenzaldehyde.

|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
follow-up questions
Could you post a wide-angle of the apparatus? Thanks, I'm getting ready to work with an inert atmosphere myself.
1) "A 100mL single-neck schlenk, connected to the argon line and equipped with a efficient condenser, magnetic stirring, and vacuum inlet was charged
with 3.63g (55.00 mmol) 85% KOH and 30mL dry DMF.."
I don't understand the KOH parameter. You said 85% and later indicated they are in the form of pellets. 85% what? (what's the other 15%?)
2) you mentioned gas flow several times. Did you not have a ballon or bubbler to maintain a steady gas head?
3) the end point chromatogram- how did it differ from the earlier? the chromatography genrally- I got lost on what was a spot and what was a "stain"
and how you differentiated. Would you please clear that up?
4) the inert technique generally- this was a good post for me to read. I'm getting ready to attempt a five step process that may or may not require
inert conditions all along. Inert was mentioned at specific points in the writeup and I didn't get product twice so I'm going to assume inert all the
way.
I was planning to use regular glassware with gas/vacuum lines and a powder addition funnel. I have to maintain the atmosphere for 72 hours so I can't
be sweeping the whole time. Could you comment on the Schenk flask, advantages, reasons for using it instead of rb's? Could you tell us about the
syringe? Was it a gas tight model? Or did you take measures to keep air out? If so what?
5) MeI- was it essential? Could Me(SO4)2 be subbed in?
Thanks for a very complete writeup. Lot's of good information here.
PS- anybody know when the MeI tox data was developed? I seem to run into older papers, from the 20's-30's that talk about making MeI in the lab
without much precaution.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Thanks for pictures! Well written! It's nice to see someone actually doing something in this silly forumlet. 
| Quote: | Originally posted by chemrox
I don't understand the KOH parameter. You said 85% and later indicated they are in the form of pellets. 85% what? (what's the other 15%?)
|
KOH is commercially available in 85% purity; the remainder is chemically combined water that cannot be removed (even on fusion I don't know...). 85%
is generally accepted as reasonable KOH.
| Quote: | | 3) the end point chromatogram- how did it differ from the earlier? the chromatography genrally- I got lost on what was a spot and what was a "stain"
and how you differentiated. Would you please clear that up? |
When you do chromatography, e.g., TLC (Thin Layer Chromotography), you plop down a teensy weensy droplet of your sample, then run solvent through the
substrate, which moves the sample through the "column", which slows down different molecules at different rates. After running for a few minutes, if
there is more than one component, it becomes obvious, if not under visible light then under UV. At school, we use silica gel TLC plates which
fluoresce green under short UV in the absence of chemicals. Where the chemicals come to rest, you see a dark spot surrounded by glowing green.
| Quote: | | 5) MeI- was it essential? Could Me(SO4)2 be subbed in? |
He said, in the conclusion,
| Quote: | | ...dimethylsulfate seems to be a much more practical reagent... |
Which implies it would do just as well, if not better.
Tim
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@12ax
Thanks for your interpretations. Having a lab myself I have a passing familiarity with TLC (my undergraduate thesis relied heavily on it) so my
questions for the author still stand. I didn't know about the KOH purity issue and will pay closer attention as needs arise.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemrox
Could you post a wide-angle of the apparatus? Thanks, I'm getting ready to work with an inert atmosphere myself. |

The argon comes from a disposable cartridge, a inline drier full of CaCl2 and Cu2O, although that's more a unnecessary precaution as the gas seems
pretty pur, no water, hardly any oxygen. For really sensible reactions (organolithiums, etc), I would obviously use a different purity, but that's not
the kind of reaction I do at home... A mineral bubbler help to see the flow of gas.
| Quote: |
2) you mentioned gas flow several times. Did you not have a ballon or bubbler to maintain a steady gas head? |
I didn't use any ballon simply because I didn't have any at hand, even though it would obviosuly be very usefull. As inert conditions weren't
rigorously needed, I prefered keeping a blanket of argon rather than a continous flow or slightly pressurized system. A piece of cotton in the CaCl2
guard helps to minimize air movements. Othewise, I usually use a small wash bottle with some mineral oil to isolate the system from the atmospher, and
avoid pressurization.
The gas flow was maintained when adding reagents to preevnt air from comming in. As soon as the MeI was added this was cut because the gas flow
would have pushed MeI vapors up the condenser.
| Quote: | | 3) the end point chromatogram- how did it differ from the earlier? the chromatography genrally- I got lost on what was a spot and what was a "stain"
and how you differentiated. Would you please clear that up? |
Sorry the confusion comes from my native language, stains and spots are the same for me :). In the first plate, there was only a rather large
spot, the top was at the same level as the starting material, but the bottom corresponded to the product. In the last plate, the large spot had
"fallen" back to were the product normally is, and there wasn't anything next to the starting material spot. I considered that the two compounds
weren't efficiently seperated by straight DCM as eluant.
| Quote: | 4) the inert technique generally- this was a good post for me to read. I'm getting ready to attempt a five step process that may or may not require
inert conditions all along. Inert was mentioned at specific points in the writeup and I didn't get product twice so I'm going to assume inert all the
way.
I was planning to use regular glassware with gas/vacuum lines and a powder addition funnel. I have to maintain the atmosphere for 72 hours so I can't
be sweeping the whole time. Could you comment on the Schenk flask, advantages, reasons for using it instead of rb's? Could you tell us about the
syringe? Was it a gas tight model? Or did you take measures to keep air out? If so what? |
When using rather sensible compounds, such as aldehdyes and phenolates that are readibly oxidized by oxygen, using a schlenk technic really is a
confort. Although it is a bit of an overkill. As I have the glassware at hand, and constantly use this technic at work, I use it at home too. But
getting all the right steps and all can be a little tricky at first, especially if there's no one to show you the first times. Basickly, the use of a
argon/vacuum manifold is essential, although I don't have one at home ($$), so things are les practical. A manifold ables you to switch from vacuum
toa rgon with the same "tap" (don't recall the name in english right know). Once you have connected you setup, you evacuate by using the vacuum for a
few minutes, then let a flow of argon come in. After reapeating this a few times, most if not all of the oxygen and water vapor present has been
disposed of. Then, under a flow of argon to avoid air from entering, you attach a rubber septum to one of your RBF neck or elsewhere, to be able to
introduce liquid reagents. This can be done using either glasss syringes, or another small schlenk and a canula (like a teflon capilary), which under
a slight pressure of argon will "push" the liquid into the setup. Each time the system is opened for one reason or another, a flow of argon is allowed
to consatntly come out the opened neck.
Not having a manifold, I connect the vacuum to the top of my condenser, and the argon to the schlenk tap. I open the vacuum tap, thne close it and
open the argon,etc etc. Once I 've purged evrything and won't be needing the vacuum anymore, and disconnect the vacuum tap and attach a septum, or a
wash bottle with oil. In some case where the inert atmospher isn't critical, like here, I can simply pulg the top with some cotton or such. The argon
in the condenser protects the schlenk's content, and a little flow of argon is use now and then to chase the air that has diffused in. But using a
ballon would be simply and more efficient of course.
concerning the syringe, it's a regular glass model, that isn't completly air tight as these syringes aren't. Usually, you sue a slight pressure of
argon to fill it up, and then a slight vacuum or simply gravity dripping to empty it. As neither the DMF or the MeI were under inert atmospher, the
liquids were simply redraw, which always leave a little air come in. The syringe is simply topped up, and the air bubbles chased out, not with the MeI
though as I wanted to manipulate this compound the minium. The little amount of air introduced wasn't problematic, though if it was the liquids would
have been distilled under agon into small schlenk, and transfered using an argon pressure into the syringe as discribed earlier. The salicylaldehdye
was transfered using a canula, it was purged in the small schlenk (applied vacuum until the liquid comes into ebullition, send argon, etc etc), and a
septum fitted, to able the use of a canula: the capilarry is fitted in the septum of the smalls chlenk, and on the other side in the septum of the
condenser, a small needle is fitted in the same septum, and a flow of argon is entered through the small schlenk. The capillary is pushed under the
surface of the solution, which is "pushed" into the setup.
| Quote: | | 5) MeI- was it essential? Could Me(SO4)2 be subbed in? |
dimethylsufate would have been prefered, as it is more efficient, less volatil and thus easier to work with.
| Quote: | | Thanks for a very complete writeup. Lot's of good information here. |
My pleasure :)
Thanks 12AX7, i hope this kind of post is stimulating enough to encourage other people to share their experiments too :)
[Edited on 9-2-2008 by Klute]
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
hey
this is a good job ! Your cristals are beautiful, I'll eat some for my breakfeast !!! and 73% is a good yield after recristallisation.
I've done the same job (O-alkylation of o-salicyladehydes) with Trimethylphosphate and K2CO3 in DMF. I had good yield, too . But bad yield with
acetone as solvent.
TMP is a good alkylating agent much less toxic than the dangerous Dimethylsulfate. I prefer TMP or MeI instead of DMS.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Yes, I've considered using TMP too, although I heard some people claiming low yields. Indeed, this compound is much safer to handle than either MeI or
DMS, and is available through pyrolysis of MeOH/P2O5/NaOH a la Halfapint.
How good were the yields? >70%? How long did you let the reaction proceed, and at what temperature? Any further details would be appreciated.
| Quote: | | Your cristals are beautiful, I'll eat some for my breakfeast !!! |
You better not! After all the effort...
I'll go and hide them right away  You can have some vanillin if you really are
hungry You can have some vanillin if you really are
hungry 
EDIT: the mother liquor of the recrysatllization was sent back to the freezer, and another small portion of crystals were obtained, as pur as the
first ones (color and TLC). The total yield is now 82.47%.
[Edited on 9-2-2008 by Klute]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@Klute-since you're doing this kind of thing at work please read and criticize my upcoming post in Org-Chem-I'd appreciate the feedback. I rigged a
three-way valve to the vacuum pump and gas supply and it worked fine but being ignorant I didn't cycle enough time and pull the vacuum down far
enough... I coluld have done and will next time around.
PS- I have a crush on the apparatus ;^)
[Edited on 9-2-2008 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Message original : Klute
How good were the yields? >70%? How long did you let the reaction proceed, and at what temperature? Any further details would be appreciated.
|
I dindn't really remember what I've done but I'm sure that refluxing the p-methoxy-salicyladehyde in acetone with K2CO3 for 48h give no yield. too
much phenolic salt are present.
But the second trial was performed by dissolving the phenolic aldehyde in DMF with 1.5 eq of TMP and K2CO3 as base and heating it to ~80°C (?) for
24h give really good yield.
When I used NaOH as base, I obtained a lot of black tar.
But The for the last trial I've prepared the K-salt of the phenolic aldehyde (maybe 4-MeO or 3-MeO) by dropping it in a 25% KOH solution. The solid is
filtered and the resulting cake washed with EtOH (~80% yield, can be better by treating it with EtOK instead of KOH). The K-salt was added in a
minimal amount of anhydrous DMF heated at 80°C (???). Then, an excess (1.5 mol per mol of the K-salt) of TMP was added to the mixture over 30 min
and the mixture was left for 1/2 day . The mixture was added to cold water and the resulting precipitate was filtered and washed with 2% NaOH and
distilled water.
The yield was very good ! >80% for sure (perhaps >90) !!!
[Edite le 12-2-2008 par Methyl.Magic]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Methyl.Magic
But the second trial was performed by dissolving the phenolic aldehyde in DMF with 1.5 eq of TMP and K2CO3 as base and heating it to ~80°C (?) for
24h give really good yield.
....for the last trial I've prepared the K-salt of the phenolic aldehyde (maybe 4-MeO or 3-MeO) by dropping it in a 25% KOH solution. The solid is
filtered and the resulting cake washed with EtOH (~80% yield, can be better by treating it with EtOK instead of KOH). The K-salt was added in a
minimal amount of anhydrous DMF heated at 80°C (???). Then, an excess (1.5 mol per mol of the K-salt) of TMP was added to the mixture over 30 min
and the mixture was left for 1/2 day . The mixture was added to cold water and the resulting precipitate was filtered and washed with 2% NaOH and
distilled water.
The yield was very good ! >80% for sure (perhaps >90) !!!
[Edite le 12-2-2008 par Methyl.Magic] |
That seems very encouraging. How much of an improvement is preparing the alkali salt ahead of time, instead of insitu? Does totally anhydrous
conditions really boost up the yields? It may be wiser to make the salt with KOH in ETOH rather than a aq. solution, EtOK is perhaps a little over
kill.
When I've finished all the projects I have already started, I will give the TMP synth from P2O5/MeOH/NaOH a try. If the yields are fairly low, I'll
try the DMS distn also. Now the other part is finding new substartes to alkylate..
I already have at hand: 2-MeOBnO, 4-MeOBnO, 3,4-MeOBnO, and of course straight BnO and vanillin; I plan on condensing them with acetone and
reducing to the saturated ketone, and see all the different sents/flavor.
But I've still a few things to finish first, and alot of cleaning up to do 
Thanks for sharing your results, you've help me regain trust in TMP 
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
For the next Job perhaps I'll try a "all-in-one" o-formylation of phénol.
1) dissolve the phenol in EtONa
2) Add some touene and distill off the MeOH
3) Formylate the phenol with PFA
4) Add some DMF and boil off the toluène
5) Go with a known method of O-methylation (TMP, MeI, ... )
All this prods have a really nice smell ! Especially Vaniline ! You should look to acetophenone analogues of known benzaldehyde or the benzoate ester
too ! 4-MeO-acetophenone smells very very nice !!! I can't imagine the analogue of vaniline !
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Methyl.Magic
For the next Job perhaps I'll try a "all-in-one" o-formylation of phénol.
1) dissolve the phenol in EtONa
2) Add some touene and distill off the MeOH
3) Formylate the phenol with PFA
4) Add some DMF and boil off the toluène
5) Go with a known method of O-methylation (TMP, MeI, ... )
|
I'm not sure this would be such a good idea. Excess paraformaldehdye can fuck up the alkylation, as well as all the inorganic salts. You should at
least wash the toluene layer after acidification a few times, dry, remove the toluene and add the DMF.
A good idea would be to follow U.S. Pat. 3,867,458, which isolates the benzaldehyde as it's phenolate in acetone or EtOAC, which can then be
directly alkylated. But these salts are very prone to atmospheric oxydation/degradation, ideally they should be handled under inert atmosphere.
BTW, I don't think sodium ethoxide would be as efficient as the original magnesium methoxide, because the magnesium ion plays a important role in
coordinating the phenolate and the formyl group. A possible mecanism is detailed in Acta. Chem. Scand. 53, (1999) 258-262, though they use
anhydrous MgCl2 and triethylamine to form the phenolate. There is also a variation of the reaction were the phenolate is formed using a equimolar
amount of Mg metal, and a light acid such a s AcOH added to neutralise half of the methoxide, obtaining a mono-phenolate magnesium salt, which
eliminates the need for totally anhydrous conditions. I haven't tried that variation yet. I will find the patent if you are interested.
Magnesium metal powder/shards are fairly cheap I would suggest their use. The reaction, although quite long and somewhat tedious, is very high
yielding and pretty straight forward. Next time I will give it a try, I will make a complete report.
| Quote: |
All this prods have a really nice smell ! Especially Vaniline ! You should look to acetophenone analogues of known benzaldehyde or the benzoate ester
too ! 4-MeO-acetophenone smells very very nice !!! I can't imagine the analogue of vaniline ! |
Yes, I definatively want to try them out. The benzoate ester would be pretty easy to make by oxidizing the aldehyde and esterification, how did you
form the acetophenones? Friedel Craft?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
The following article, kindly retreived by Solo,
Mg-mediated o-specific formylation and formaldoximation of phenols
R. Aldred, R. Johnston, D. Levin, J. Neilan
J. Chem. Perkin Trans. (1994) 1823-31
clearly states that Mg(OMe) cannot be replaced by alkali cations with the same results:
| Quote: |
Use of less tightly coordinating cations ( e.g. Li+, Na+ or K+) in place of the small highly charged Mg2+, does result in the first
addtion step [formation of the benzylic alkoxide](albeit without the ortho-specificity observed with Mg2+) but this is followed by
elimination of hydroxyl and formation of phenol-formaldehyde resins via quinone methides in preference to the redox step [formation of
the aldehdye] giving the salicylaldehdyes, which predominates in the presence of the methanol solvated Mg2+ counter-ion. |
So the use of mg methoxide really seems necessary for this reaction. If Mg metal isn't availble, anhydrous MgCl2 can be used with triethylamine,
although the anhydrous salt would be more expensive than mg turning/powder. SnCl2 can be used with butylamine IIRC.
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Just want to add, I've done this reaction a few times and it does work quite well (on 4-methoxyphenol). However, a few snafus should be noted:
The product 2-hydroxy-5-methoxybenzaldehyde does not appear to form an adduct very well at all; I would expect this to apply to other phenolic
aldehydes as well.
If you go the Mg(OCH3)2 route as per the Aldred paper (that Klute mentions above), be sure to follow the instructions *exactly*. Subsequent analysis
found that if you use a lower temperature to perform the formylation, you get a low yield (40-50%), whereas if you go too high the aldehyde starts
charring a little bit.
The 95*C (off the top of my head, I believe that's what it is) temperature used in the paper works very well. If the phenolic aldehyde is not your
intended product, then alkylating and subsequently purifying via the sodium bisulfite adduct works *very* well.
The easiest way to run the reaction is to use a 3-neck flask, with reflux condensor, thermometer, and an empty stopper. As the reaction proceeds,
methanol is generated which lowers the temperature of the reaction mixture (which should be held at 95*C, or as close as possible) - in the paper they
distill it off, but I have found it easier to merely open the stopper and let it evaporate briefly (10 seconds or so) until the temperature increases
to 95*C again. In this manner, you can keep the reaction at 95*C +-.5*C.
Other than that though, this reaction seems to work very, very well!
Edit: Want to add: this reaction seems to be OK with a small amounts of impurities (in the phenol) - that is, it will work but only OK. I think using
a substrate with more than 5-10% impurity, the aldehyde must be isolated as an intermediate and not used directly for the next reaction (which may be
done otherwise if the reaction works properly).
[Edited on 27-2-2008 by PainKilla]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I'm glad to here other had good results with this reaction! I'm surprised not much people looked into it.
There a explanation given in that very informative, well written article for diminshing yields with lower temperatures, as when the
methanol/Mg-phenolate ratio increases, the hydride transfert accures much less easily, and the formed benzylic alkoxides gives so polymeric mess with
the formaldehdye.
I've also witnessed that the salicyladehdyes don't react with bisulfite. I think this is du to hydroxgen bonding between the phenol fonction and the
carbonyl, "protecting" the C=O from an nucleophilic attack by the rather large bisulfite anion.
| Quote: | Originally posted by Painkilla
If the phenolic aldehyde is not your intended product, then alkylating and subsequently purifying via the sodium bisulfite adduct works *very* well.
|
Do you mean alkylating the crude oil obtained afetr work up and evaporation of the solvant, or direct alkylation of the mg phenolate? The excess
formaldehdye might be problematic, don't you think?
On the second hand I recall a patent purifying the obtained salicylaldheydes by forming the alkali phenolates by adding conc. alkali hydroxides
solutions to the crude product in acetone or EtOAc, which can be directly alkylated after filteration by known means in good to great yields. These
phenolates salts are very prone to atmospheric oxydation IIRC.
Painkilla, as mentionned in several patents, the distillate of the recation can be recylced when preparing another batch of magnesium methoxide.
This could be safer than breathing toluene/methanol fumes, aswell as formaldehyde and associated compounds  The vapor temperature also ables one to follow the composition of the distillate: as soon as a portion of
paraformaldehyde is added, increased boiling and decrease in distillate temp follows. The vapor temperature also ables one to follow the composition of the distillate: as soon as a portion of
paraformaldehyde is added, increased boiling and decrease in distillate temp follows.
A few things surprise me: The yellow/orange suspension clears up to a limpid orange solution whe roughly 2/3 of the paraformaldehyde has been added.
I interpreatted that as being to du to the fact that the hydromethylated compound is formed at first, then dehydrogenation accurs. This could be
plausible as hydroxymethylation is independant of the amount of MeOH present, whereas formation of the salicyladehyde requires a MeOH/Phenolate ratio
inferior to 2, which would arrive after most of the MeOH has distilled.
But the authors of the article claim that the starting mg phenolate i soluble in toluene. Indeed, the slurry formed becomes less thick as more
tolune is added, but still remains a thickish suspension. What could it be then? The benzylic alkoxide?
Also, at the end of the PF addtion, the reaction mixture is a limpid orange solution. But an hour after, it becomes a suspension as the apparent mg
phenolate precipitates. How come this accurs only a hour after?
[Edited on 27-2-2008 by Klute]
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
I mean isolating the aldehyde from toluene post-acidification of the reaction mixture, and then alkylating without any further purification steps.
I have my doubts that the magnesium phenoxide would be possible to alkylate at all; toluene will not work for this first of all, and second, it's so
easy to isolate the aldehyde there's really no reason to alkylate the magnesium phenoxide to begin with.
That patent does not work without an inert atmosphere (if at all). The aldehyde oxidises extremely rapidly, making isolating near impossible. If one
were to do it under nitrogen or argon, I think this would then work OK, but it's still better to use the adduct if you can get it to work.
I initally performed the reaction before viewing the paper (and subsequent details about temperatures effect on yield.) I had thought I isolated my
product and yield was as advertised - but subsequent alkylation gave very low yields, and it was only after analysis that it was realized that impure
2-OH-5-MeOBzA is very similar to pure aldehyde. This might not be the case with other aldehydes, but should still be looked out for (IE, NMR TLC etc
is a good idea).
Inhaling vapors is not good, that's why one should use a hood, or read a new book outside when the reaction is going. 
I've personally have not noticed the phenoxide precipitate. The reaction starts off pale yellow from what I remember, and then turns a yellow-orange,
which then turns yellow upon workup. I don't recall seeing any precipitation at all at any point in the reaction. It also appears to me to have been a
gradual color change, not an "immediate" one... maybe it's just the particular substrate?
Edit: How significant is hydrogen bonding in water though? It seems to me that this would only play a (significant) role in aprotic solvents. This
would mean that it should still be relatively possible to form the adduct should one let it react long enough. I did not let it react for an overly
long time, so this may have been the problem.
[Edited on 27-2-2008 by PainKilla]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Painkilla
I have my doubts that the magnesium phenoxide would be possible to alkylate at all; toluene will not work for this first of all, and second, it's so
easy to isolate the aldehyde there's really no reason to alkylate the magnesium phenoxide to begin with.
|
Yes, I agree with you. I misunderstood your last post and thought that was what you had planned.
| Quote: | | That patent does not work without an inert atmosphere (if at all). The aldehyde oxidises extremely rapidly, making isolating near impossible. If one
were to do it under nitrogen or argon, I think this would then work OK, but it's still better to use the adduct if you can get it to work.
|
What adduct? Bisulfite? I'm lucky enough to be posses schlenk glassware, and could do a schlenk filteration of the phenoxide and subsequent
alkylation, but obviously if alkylating the crude product works well, no need to put so much effort into it.
What alkylating system did you use? What were the yields?
| Quote: | | [...]subsequent alkylation gave very low yields, and it was only after analysis that it was realized that impure 2-OH-5-MeOBzA is very similar to
pure aldehyde. |
by the pure aldehyde, to you mean the alkylated aldehyde? The methylated aldehyde is a solid at RT IIRC, and the salicylaldehyde a oil, no? Or do
you mean that the crude product was actually quite impur even though it "looked" pur? What kind /how much contaminants did you encounter? I could only
detect what seemed to be the dimeric impurity on the crude products.
| Quote: | | I've personally have not noticed the phenoxide precipitate. The reaction starts off pale yellow from what I remember, and then turns a yellow-orange,
which then turns yellow upon workup. I don't recall seeing any precipitation at all at any point in the reaction. It also appears to me to have been a
gradual color change, not an "immediate" one... maybe it's just the particular substrate? |
Oh well, I wanted to keep these photos until I reconducted the reactions to complet them, and make a dedicated thread, but I can't resit anymore  . Obviously, I will make a new thread when the next reaction is performed. I will
leave this thread for other alkylations in the futur. . Obviously, I will make a new thread when the next reaction is performed. I will
leave this thread for other alkylations in the futur.
Formation of magnesium methoxide

Reaction just started, initiated with a crystal of I2
Formation of the phenolate

After addition of the phenol in toluene. At the begginign of the addtion, the thick precipitate made stirring delicate, but the sludge thinned up with
strong stirring. Adding a certain amount of toluene before adding the solution could avoid this.
Addition of the paraformaldehyde


Just after adding the first portion. Notice the yellow color appearing.
Half-way through addition:

1 hour after the end of the addition, just before acidification:

The solids on the sides are the phenolate, and not any excess paraformaldehdye which depolymerise in a matter of seconds, visually, in such a medium.
I will make a more detailed report when I have more pictures, as I didn't take any during workup.
Painkilla, how well did you dry your reagents? I had the feeling strict anhydrous conditions would be needed for good yields, and I was surprised to
see this wasn't really mentionned in the article above.
My home-made paraformaldehyde (vac. distn of 31% Formalin) was dried over NaoH for a few days, or P2O5. I will be using lab grade next time. The
phenol was also powdered and dried over NaOH for a few dasy, and all solvants dried a few days over 3A mol sieves.
Home-made, dried paraformaldehyde.

|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Forgot to add... awesome work! 
It is the bisulfite adduct that I speak of. The most effective way I've found to do this is to, immediately after the time passes for alkylation (on
the crude aldehyde), add toluene and water, shake up the mixture to allow the phenoxide to move into the water layer, then separate the toluene and
dry with a suitable agent (I typically used Na2SO4). The toluene/solvents are then evaporated under vacuum until most of the solvent is removed (you
want the aldehyde to be fairly concentrated in the toluene), and concentrated sodium bisulfite solution added, maybe 3-4x the molar excess of the
theoretical amount of aldehyde. This is then stirred for a few hours, and you get a nice layer of clear/off white crystals which are not at all dense.
These are then vacuum filtered, and the adduct decomposed in chilled 10% hydroxide solution, upon which the aldehyde crystallizes nicely out of
solution and you can then vacuum filter dry etc...
I used acetonitrile/DMS/K2CO3 to methylate, with yields being about 70-80%. The reagents for the methylation were dry, but not EXTREMELY dry, so that
might be why they are a bit lower than they "should" be. But overall, it works quite well.
When the reaction is properly performed on 4-methoxyphenol you get substantially pure product. When the reaction is done at a lower temperature you
get a product that crystallizes at the same temperature as 2-hydroxy-5-methoxybenzaldehyde, smells just as wonderful (seriously, one of my favorite
compounds, I can't help but to rub a bit on my hands ((which then get stained yellow)) so that I may then smell them throughout the day), and has
otherwise the same appearance. TLC of course, shows otherwise. I did not run the analytical portion myself, I gave a sample to a friend who determined
that the aldehyde was only ~50% pure.
Additionally, methylations with that impure compound initially gave me much frustration, because I couldn't figure out what the low yields were caused
by, but after realizing it was the product of the formylation that was impure, rather than the methylation failing, the methylation started to give
yields appropriate for that reaction.
That said, I also tried methyl iodide and acetone, and if I remember correctly, acetonitrile and methyl iodide. The yields relative to DMS were less,
but taken in the context that I may have had (other) phenolic impurities in the reaction, the yields were likely similar to DMS, or very slightly
lower.
As far as the reagents for the formylation, I used commercial grade paraformaldehyde which, to my knowledge was fairly dry. I can't attest to how dry
it truly was, but it seemed to OK. That aside, methanol was dried with molecular sieves (3A IIRC) before reacting with magnesium, and the toluene was
dried over sodium sulfate and distilled to cleanup any gunk in it. I've also run the reaction with hardware toluene as is, and that worked just as
well. Realize that the magnesium methoxide is quite capable of scavenging stray water molecules, so you effectively have an "in situ" drying agent. As
a result, I believe to attain a reasonably dry reaction, save the dryness of the paraformaldehyde, one only needs to let the toluene and added phenol
stir with the magnesium methoxide for a little while. If you used an excess of methanol initially, the water should evaporate anyway (as you distill
to get the appropriate temperature).
The reaction does appear to be fairly similar to what I had. Which aldehyde is that by the way?
[Edited on 27-2-2008 by PainKilla]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
That reaction was with unsubstitued phenol, and gave 84% IIRC, after steam distn of the aldehyde, which was long, tedious, and unnecessary.
There si a variation of this reaction where an equimolar amount of Mg is used, and an acid added after addtion of the phenol, to obtain
mono-phenoxide salts, instead of the bis-phenoxide. Apparently, this eliminates the need for dry conditions, and diminishes the extent of
polymerisation.
But using twice as much magnesium metal might not be worth it. I always used an excess of Mg anyway to keep dry condtions as you mentionned.
This is IMHO a very interesting subject/reaction, would you be inclined to share your experience with this reaction in detail in a dedicated
thread? I'm planning on doing the recation as soon as I receive the paraformaldehyde, in less than a week. We could make a new thread, and transfert
most of the discussion other there.
Going back to the original thread subject, I will also try out TMP s a safe methylating agent, as soon as my bottle arrives, with the
paraformaldehyde. Although (much) more expensive than DMS, i feel much more confortable having a bottle of TMP around and using it rather than having
a liter of DMS which would raise avok if by any means it would break, and would in any case be more than what I would need in a life's time.
On the other hand, that means that I will surely not try the TMP synth from P2O5, nor the DMS synth from MeOH/H2SO4, at least in near futur.
As advised by Methyl.Magic, I will try it out in DMF with K2CO3 as a base, on salicylaldehyde. Pictures will follow  . .
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Yeah, I agree this reaction is a very overlooked method of formylation.
There are actually a variety of substrates that I will be putting through using this reaction at some point in the future, though I am not sure of
when this will actually happen. In any case, I think the reaction works quite well as it is described in the paper, and if anything should be further
tuned to specific substrates rather than as a whole.
Personally, I think DMS is really a fairly safe methylating agent - it is non-volatile, requires mild conditions to use, and is most importantly
effective and easy to scale - thereby avoiding you constant contact with these rather unpleasant to work with chemicals.
I was under the impression that DMS comes in PVC bottles for specifically the reason mentioned: spilling a bottle means death. Or at least a very
upset professor. Or possibly building of professors.
In any case, this reaction is a VAST improvement over the Reimer-Tiemann, although it's efficiency definitely falls with more hindered phenols.
However, I think we should save discussion for the other thread.
What is perhaps more interesting (returning to the focus of the thread) is that methyl iodide can be used to selectively methylate certain phenols,
such as 5-hydroxyvanillin in fairly good yield. I am sure there are other applications where this effect could be greatly utilized.
As an(other) interesting aside, I tried a few times to use the Crossed Cannizzaro reaction to form the benzyl alcohol, but it failed. Further looking
into the matter, it seems that 2-hydroxybenzyl alcohols quite easily polymerize (with formaldehyde). Kind of unfortunate - but an easy way to make
plastics (or crap :p ).
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Update: Now that more salicylaldehyde is at hand (see formylation thread [EDIT: moved to prepublication forum]), I will try out the methylation with
TMP in DMF with K2CO3 as a base, as stated by MethylMagic.
Detailed writeup to come!
[Edited on 11-3-2008 by Klute]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Let me say TMP is a breeze to work with compared to the othe alkylating agents!
The product is recrysatllizing as I type, so the final yield isn't determined yet, but a 92.08% yield of crude product was obtained!
Methylation of salicylaldehyde with Trimethylphosphate in DMF in presence of excess K2CO3
Experimental
In a purged 250mL 4-neck RBF, with condenser, addition funnel, argon and vacuum inlets, magnetic stirring and thermometer, 18.3g (132.4 mmol) of
freshly fused K2CO3 are introduced, followed by 50mL of dry DMF via syringe. The whole systeme is briefly degassed, and maintained under slight argon
over-pressure.
12.21g (100 mmol) of 2-hydroxybenzaldehyde (salicylaldehyde) are placed in a 100mL schlenk, followed by 20mL DMF. The flask is evacuated several
times until ebullition (degassed), and maintained under argon. Using a canula, the yellow/green solution is transfered to the addition funnel through
a septum.
The aldehdye solution is then slowly dripped into the K2CO3 suspension, a bright yellow/orange colour immediatly appearing. Once all the solution
has been added, resulting in a pumpkin bright orange suspension, the schlenk and addition funnel are rinsed with 2*10mL degassed DMF. Stirring is
continued at room temp for 10min.
17.55mL (150 mmol) of trimethylphosphate are added into a 100mL schlenk, 10mL DMF added, the solution degassed and transfered to the addition
funnel, and finally dripped in the bright orange suspension over 30min, not noticeable colour change occuring. Heating up to 80°C with an oil bath is
started.
After 1h, the colour has darkened to a ark orange/brown.
Heating is maintained for 20H, afetr which a dark brown susspension is obtained. The flask is left to cool for 1H, and the reaction mixture drowned
into 200mL dH2O. A voluminous brown precipitate appears. STirring with a glass rod occasionally, the beaker is left to rest for 10min, and the
contents vacuum filtered. A light brown cake is obtained, which is triturated with 2x50mL dH2O on the buchner, loosing some color, and transfered to
a 500mL beaker. 150mL of 1% NaOH is then added, and the thickish suspension triturated for 5min, then vacuum filtered. A light pink/beige cake is
obatined, it is triturated with 2x100mL dH2O, until washings are neutral, then dried by suction for 10min.
Another smaller batch of solids are obtained from the first filtrate, and treated in a similar way.
The crude product weighs 14.1g after drying for 1H over NaOH.
It is dissolved in 25mL AcOEt, giving a dark red solution, filtered through a cotton to remove some insoluble black impurities, and placed in a
erlenmeyer. 50mL of pet. ether are evry slowly added on the sides of the erlenmeyer, giving two layers. After 2min, large needles start to form.
The crystals will be filtered tomorow, yield determined, and pictures posted.
Comments:
Obviously the use of inert atmosphere isn't mandatory. I used out of precaution especially when forming the phenolate, as it is pretty sensitive.
I'm pretty sure shorter reaction times are needed. I didn't use TLC to follow the reaction, as I wasn't there during most of the heating. Shorter
reaction times and possibly lower temperatures might avoid the dirty byproducts. I must admit the reaction with MeI is much cleaner. But the change is
worth it.
Cheers to MethylMagic for sharing his experience, the reaction conditions were choosen according to his comments. Thumbs up!
I've placed that MeI bottle far far in the back of the reagent cupboard, and don't plan on letting out in near futur 
[Edited on 13-3-2008 by Klute]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Humm... too late to edit.... Sorry for the 3 posts in a row.
Continuation:
The crude product was placed in a wide neck erlenmeyer, and covered with 50mL pet ether. No dissolution or colour take-up was noticed. With
stirring, ~20mL of AcOEt was added dropwise; the pet ether gradually took on a red wine colour. The solution was filtered through a small piece of
cotton to remove some black insoluble impurities, and 30mL of pet ether were very carefully and slowly added on the sides of the erlenmeyer, taking
care to not disturb the two layers, and everything left to rest overnight. Very large needles had formed in the morning.
These were filtered, and washed with a little pet ether, and dried by suction. Some fluffy solids appeared in the filtrate, so it was slightly
heated to acheive total dissolution, and replaced in the erlenmeyer. The erlenmeyer was then covered and placed in the fridge for 2H, were more big
needles formed, and then in the freezer. After another hour, these crystals were equally filtered, washed and dried. The combined crystals were left
in a CaCl2 dessicator, and weighed 11.04g (72.09 mmol), giving a 72.09% yield.
There still is some product left in the filtrate, as crystals form when a little is evaporated, so it was left to evaporate partially at room temp.
Should have used less pet ether from the start, and possibly a little less AcOEt. DCM works very well, if not better, but none was availble at the
time of the recrystallization (got to redistill the used solvant).
Although nicely recrysatllized, the crystals are less pur than the MeI run (notice the difference in color), as they give a dark red solution when
dissolved. No TLC yet, waiting to get the fresh DCM. A second recrystallization would be usefull, but for the condensations I don't think it will be
worth it.
Shorter reaction times would surely give a cleaner product.
Pictures:
The setup:

Salicylaldehyde in schlenk:

Transfering the sal solution:

After adding the first few drops of sal to the K2CO3 suspension:

All the aldehdye added:

The friendly methylating agent  : :
Addition of TMP:

After 20H heating:

After drowning the reaction medium in water:

Crude product:

Recrystallization:
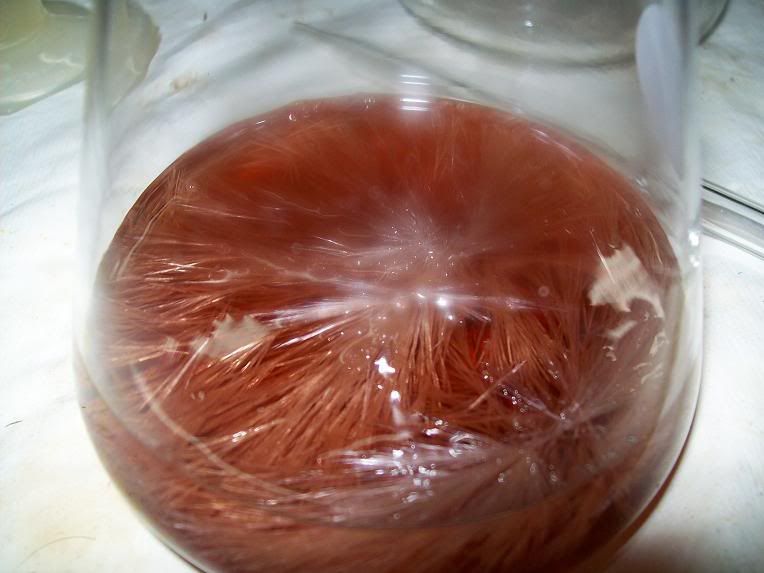
First batch of crystals:

Second crop:

Combined crystals:

All in all, TMP is definatively a good alkylating agent. I'm pretty sure the yields would be even better with more activated cycles. Although TMP
is much less noxious then either MeI or DMS, minimal precautions must be taken. I personally kept the gas mask on as I don't have a hood.
I'm sure there's room for a little improvement, as the product is much les cleaner than with MeI. A bisulfite purification could be adviseable, but
I would prefer doing without.
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
Bien Joué ! Ca c'est du bon boulot !
I'm happy to see that TMP alkylation gives good yield !
So, the next week-end I'll start the megnesium methoxide formylation of 4-MeO-Phenol to syth 2-HO-5-MeO-Benzaldehyde. The problem is that I don't have
nitrogen inert athmosphere...
I'm waiting for my new 350ml reactor !
PS : TMP can probably alkylating free amines without the formation of tetraalkylammonim salts.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Merci!
You can surely buy a argon cartridge for soldering in any large hardware store... Be carefull, the so-called "Atal" is actually 70% argon-30% CO2.
I have found that most of the argon bottles deliver a relatively dry, CO2-free gas. For reaction necessity really dry conditions, you can pass the
gas through CaCl2/NaOH.
I would say that inert atmospher would be very helpfull to avoid oxydation of the phenolate during addition of the PF, but you get around without
it.
Please report your results back, in the formylation thread!
|
|
|
| Pages:
1
2 |
|