Reflux setups
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
Materials necessary for setting up a system in reflux are an absolute must for any home lab. However, the definition of a reflux setup can range from a simple chamber that is hot at one end and cold at another, to much larger installations involving lots of glassware.
Improvised setups
One of the simplest and most commonly encountered reflux setups is a beaker full of the necessary reactants in solution or in a liquid state, with a heating source beneath the beaker and a cold surface, often a round-bottom flask of ice and water on top. Despite being such a simple setup, this reflux system is a powerful tool for aiding reactions such as esterifications or hydrolyses. This same setup is useful for sublimating a compound at the bottom of a beaker and depositing it on top of the upper container, such as in the purification of iodine.
A simple setup which is almost professional is the usage of a simple flask attached to a condenser placed on top (and clamped in place with a wide-holed rubber stopper). If a condenser is not owned, a wide (~0.75 to 1.25 cm) glass tube can be attached to the top of the flask. In this set-up, it is assumed that the length of the condenser or tubing will be sufficient to air cool and re-condense the gas back into the container. A retort turned its "nose" up can also serve as a reflux setup.
Professional setups
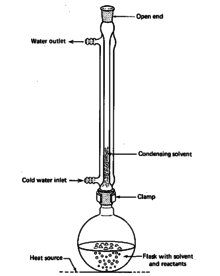
Reflux condensers or other condensers are usually used for more efficient and well-contained refluxes. These use ground glass joints to ensure no gas can escape through the joint, it all must travel through the condenser before exiting. This can greatly decrease losses, especially for volatile compounds. A setup for essential oil extractions from organic matter can ideally be done using both a reflux condenser and a Soxhlet extractor.