Difference between revisions of "Halogen"
(Created page with "The halogens on the Periodic TableThe '''halogens '''are the elements making up column 7A (or 17) of the Periodic Table. They are fluorine, chl...") |
|||
| (9 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:Halogens.png|thumb|The halogens on the Periodic Table]]The '''halogens '''are the elements making up column 7A (or 17) of the [[ | + | {{Stub}} |
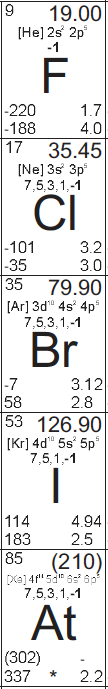
| + | [[File:Halogens.png|thumb|The most significant halogens on the Periodic Table]] | ||
| + | The '''halogens '''are the elements making up column 7A (or 17) of the [[periodic table]]. They are [[fluorine]], [[chlorine]], [[bromine]], [[iodine]], [[astatine]], and tennessine. | ||
| + | |||
==Elements in the Halogen Group== | ==Elements in the Halogen Group== | ||
===Fluorine=== | ===Fluorine=== | ||
[[Fluorine]], atomic number 9, is an extremely reactive, faint yellow gas under standard conditions. | [[Fluorine]], atomic number 9, is an extremely reactive, faint yellow gas under standard conditions. | ||
| + | |||
===Chlorine=== | ===Chlorine=== | ||
[[Chlorine]], atomic number 17, is a reactive, faint greenish-yellow gas under standard conditions. | [[Chlorine]], atomic number 17, is a reactive, faint greenish-yellow gas under standard conditions. | ||
| + | |||
===Bromine=== | ===Bromine=== | ||
[[Bromine]], atomic number 35, is a reactive, volatile, reddish brown liquid under standard conditions. | [[Bromine]], atomic number 35, is a reactive, volatile, reddish brown liquid under standard conditions. | ||
| + | |||
===Iodine=== | ===Iodine=== | ||
[[Iodine]], atomic number 53, is a reactive, volatile, black solid under standard conditions. | [[Iodine]], atomic number 53, is a reactive, volatile, black solid under standard conditions. | ||
| + | |||
===Astatine=== | ===Astatine=== | ||
| − | Astatine, atomic number 85, is still largely unobserved, because of its extreme radioactivity, but the limited studies of it have confirmed that it seems to behave similarly to the other halogens. It is predicted that it would look like a darker, denser, more metallic version of iodine, if it didn't vaporize itself with the heat from its radiation.<ref>http://en.wikipedia.org/wiki/Astatine</ref> | + | [[Astatine]], atomic number 85, is still largely unobserved, because of its extreme radioactivity, but the limited studies of it have confirmed that it seems to behave similarly to the other halogens. It is predicted that it would look like a darker, denser, more metallic version of iodine, if it didn't vaporize itself with the heat from its radiation.<ref>http://en.wikipedia.org/wiki/Astatine</ref> |
| − | === | + | |
| − | + | ===Tennessine=== | |
| + | Tennessine is element number 117. As of 2015, A total of 15 tennessine atoms have been created: six when it was first synthesized in 2010, seven in 2012, and two in 2014. It is even more radioactive than astatine. | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
The halogens are generally considered to be reactive, because they have 7 valence electrons and react with most other elements under the right conditions. They will also react with each other to form [[interhalogen]]s. All of the halogens are diatomic. | The halogens are generally considered to be reactive, because they have 7 valence electrons and react with most other elements under the right conditions. They will also react with each other to form [[interhalogen]]s. All of the halogens are diatomic. | ||
| + | |||
===Physical=== | ===Physical=== | ||
| − | All of the halogens are [[volatile]]. Fluorine and chlorine exist as gases, while bromine and iodine are readily volatile and release a gas phase. All of them are colored, with the color becoming increasingly darker going down the group.<ref>http://en.wikipedia.org/wiki/Halogen</ref> | + | All of the halogens are [[Volatility|volatile]]. Fluorine and chlorine exist as gases, while bromine and iodine are readily volatile and release a gas phase. All of them are colored, with the color becoming increasingly darker going down the group; astatine and tennessine are theoretized to be black or bluish-black, if there only was a way to collect enough of them to observe the color and not vaporize the samples due to radiothermal energy.<ref>http://en.wikipedia.org/wiki/Halogen</ref> |
| + | |||
==Safety== | ==Safety== | ||
The halogens must all be handled with care and proper [[personal protective equipment]]: rubber gloves, a respirator or gas mask (if not using a fume hood), and a lab apron or lab coat. They are toxic, corrosive, and volatile substances. Use of a fume hood is highly recommended. Fluorine should simply not be used in an amateur setting, because it ''will'' ''kill you'' more likely than not. Iodine is not subject to many of these precautions. See the safety section of each halogen's page for more specific details. | The halogens must all be handled with care and proper [[personal protective equipment]]: rubber gloves, a respirator or gas mask (if not using a fume hood), and a lab apron or lab coat. They are toxic, corrosive, and volatile substances. Use of a fume hood is highly recommended. Fluorine should simply not be used in an amateur setting, because it ''will'' ''kill you'' more likely than not. Iodine is not subject to many of these precautions. See the safety section of each halogen's page for more specific details. | ||
| + | |||
| + | ==See also== | ||
| + | *[[Interhalogen]] | ||
==References== | ==References== | ||
<references/> | <references/> | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=31696 The smell of halogens] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=7064 The Halogens and Alkalis Confined! (Pictures)] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=28730 Reaction of halogen ketones with ammonia] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=33932 Washing a halogen contaminated aromatic aldehyde with bisulfite?] | ||
| + | |||
| + | [[Category:Elements]] | ||
| + | [[Category:Nonmetals]] | ||
| + | [[Category:Halogens]] | ||
| + | [[Category:P-block]] | ||
Latest revision as of 19:00, 23 December 2021
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
The halogens are the elements making up column 7A (or 17) of the periodic table. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine.
Contents
Elements in the Halogen Group
Fluorine
Fluorine, atomic number 9, is an extremely reactive, faint yellow gas under standard conditions.
Chlorine
Chlorine, atomic number 17, is a reactive, faint greenish-yellow gas under standard conditions.
Bromine
Bromine, atomic number 35, is a reactive, volatile, reddish brown liquid under standard conditions.
Iodine
Iodine, atomic number 53, is a reactive, volatile, black solid under standard conditions.
Astatine
Astatine, atomic number 85, is still largely unobserved, because of its extreme radioactivity, but the limited studies of it have confirmed that it seems to behave similarly to the other halogens. It is predicted that it would look like a darker, denser, more metallic version of iodine, if it didn't vaporize itself with the heat from its radiation.[1]
Tennessine
Tennessine is element number 117. As of 2015, A total of 15 tennessine atoms have been created: six when it was first synthesized in 2010, seven in 2012, and two in 2014. It is even more radioactive than astatine.
Properties
Chemical
The halogens are generally considered to be reactive, because they have 7 valence electrons and react with most other elements under the right conditions. They will also react with each other to form interhalogens. All of the halogens are diatomic.
Physical
All of the halogens are volatile. Fluorine and chlorine exist as gases, while bromine and iodine are readily volatile and release a gas phase. All of them are colored, with the color becoming increasingly darker going down the group; astatine and tennessine are theoretized to be black or bluish-black, if there only was a way to collect enough of them to observe the color and not vaporize the samples due to radiothermal energy.[2]
Safety
The halogens must all be handled with care and proper personal protective equipment: rubber gloves, a respirator or gas mask (if not using a fume hood), and a lab apron or lab coat. They are toxic, corrosive, and volatile substances. Use of a fume hood is highly recommended. Fluorine should simply not be used in an amateur setting, because it will kill you more likely than not. Iodine is not subject to many of these precautions. See the safety section of each halogen's page for more specific details.