Difference between revisions of "Hexanitrohexaazaisowurtzitane"
(Created page with "{{Chembox | Name = Hexanitrohexaazaisowurtzitane | Reference = | IUPACName = 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.0<sup>3,11</sup>.0<sup>5,9</sup>]dod...") |
Diachrynic (Talk | contribs) m |
||
| Line 126: | Line 126: | ||
==Preparation== | ==Preparation== | ||
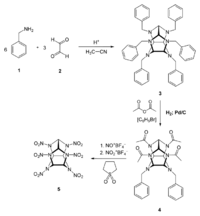
| − | [[File:Synthesis of CL-20 drawing.png|200px|thumb| | + | [[File:Synthesis of CL-20 drawing.png|200px|thumb|right|Synthesis of CL-20]] |
First, [[benzylamine]] (1) is condensed with [[glyoxal]] (2) under acidic and dehydrating conditions, using [[acetonitrile]] as solvent to yield the first intermediate compound (3). Four benzyl groups selectively undergo hydrogenolysis using [[palladium on carbon]] and [[hydrogen]]. The amino groups are then acetylated during the same step using [[acetic anhydride]] as the solvent. (4). Finally, compound 4 is reacted with nitronium tetrafluoroborate and nitrosonium tetrafluoroborate, resulting in HNIW.<ref>https://link.springer.com/article/10.1007%2Fs10573-005-0014-2</ref> | First, [[benzylamine]] (1) is condensed with [[glyoxal]] (2) under acidic and dehydrating conditions, using [[acetonitrile]] as solvent to yield the first intermediate compound (3). Four benzyl groups selectively undergo hydrogenolysis using [[palladium on carbon]] and [[hydrogen]]. The amino groups are then acetylated during the same step using [[acetic anhydride]] as the solvent. (4). Finally, compound 4 is reacted with nitronium tetrafluoroborate and nitrosonium tetrafluoroborate, resulting in HNIW.<ref>https://link.springer.com/article/10.1007%2Fs10573-005-0014-2</ref> | ||
Revision as of 15:28, 19 June 2019
| Names | |
|---|---|
| IUPAC name
2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.03,11.05,9]dodecane
| |
| Other names
2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane
CL-20 HNIW Octahydro-1,3,4,7,8,10-hexanitro-5,2,6-(iminomethenimino)-1H-imidazo[4,5-b]pyrazine | |
| Properties | |
| C6H6N12O12 C6N6H6(NO2)6 | |
| Molar mass | 438.1850 g/mol |
| Appearance | Yellow solid[1] |
| Odor | Odorless |
| Density | 2.044 g/cm3 |
| Melting point | 230–260[2] °C (446–500 °F; 503–533 K) (decomposition) |
| Boiling point | 244 °C (471 °F; 517 K) (decomposes)[3] |
| Insoluble | |
| Hazards | |
| Safety data sheet | None |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hexanitrohexaazaisowurtzitane, also called HNIW and CL-20, is a nitroamine explosive with the formula C6H6N12O12.
Contents
Properties
Chemical
HNIW will form cocrystal products with HMX[4] and TNT[5]. The cocrystal product with TNT has twice the stability of CL-20—safe enough to transport, but when heated to 136 °C the cocrystal may separate into liquid TNT and a crystal form of CL-20 with structural defects that is somewhat less stable than CL-20.
HNIW detonates releasing carbon dioxide, water and nitrogen gases.
Physical
HNIW is a yellowish solid, soluble in organic solvents.[6]
Explosive
CL-20 has a better oxidizer-to-fuel ratio than conventional HMX or RDX. It releases 20% more energy than traditional HMX-based propellants, and is widely superior to conventional high-energy propellants and explosives. HNIW has a very high detonation velocity, of 9.38 km/s. It has an OB of -11%.
Availability
This compound is not sold and currently is still under research.
Preparation
First, benzylamine (1) is condensed with glyoxal (2) under acidic and dehydrating conditions, using acetonitrile as solvent to yield the first intermediate compound (3). Four benzyl groups selectively undergo hydrogenolysis using palladium on carbon and hydrogen. The amino groups are then acetylated during the same step using acetic anhydride as the solvent. (4). Finally, compound 4 is reacted with nitronium tetrafluoroborate and nitrosonium tetrafluoroborate, resulting in HNIW.[7]
Projects
- Rocket propellant
- Explosive material
- Shaped charges
Handling
Safety
There is limited information regarding the toxicity of this compound. Studies so far have shown to possess a similar toxicity to other nitroamine compounds.
Storage
Best to use it as soon as possible, rather than store it.
Disposal
Controlled incineration or detonation is sufficient.
References
- ↑ Latypov, Nikolaj V.; Wellmar, Ulf; Goede, Patrick; Bellamy, Anthony J.; Organic Process Research and Development; vol. 4; nb. 3; (2000); p. 156 - 158
- ↑ Nielsen, Arnold T.; Chafin, Andrew P.; Christian, Stephen L.; Moore, Donald W.; Nadler, Melvin P.; Nissan, Robin A.; Vanderah, David J.; Gilardi, Richard D.; George, Clifford F.; Flippen-Anderson, Judith L.; Tetrahedron; vol. 54; nb. 39; (1998); p. 11793 - 11812
- ↑ Zhang, Chaoyang; Yang, Zongwei; Zhou, Xiaoqing; Zhang, Chenghua; Ma, Yu; Xu, Jinjiang; Zhang, Qi; Nie, Fude; Li, Hongzhen; Crystal Growth and Design; vol. 14; nb. 8; (2014); p. 3923 - 3928
- ↑ https://pubs.acs.org/doi/10.1021/cg3010882
- ↑ https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201104164
- ↑ Latypov, Nikolaj V.; Wellmar, Ulf; Goede, Patrick; Bellamy, Anthony J.; Organic Process Research and Development; vol. 4; nb. 3; (2000); p. 156 - 158
- ↑ https://link.springer.com/article/10.1007%2Fs10573-005-0014-2