Nitrate
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
Nitrate is a polyatomic ion with the molecular formula NO3−. Nitrates are salts of nitric acid.
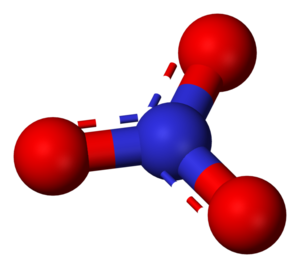
Structure
The nitrate ion consists of one nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1, which results from a combination formal charge in which each of the three oxygens carries a −2/3 charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion.
Preparation
The cheapest way to obtain nitrates is from composted manure and urine. There are a couple of methods for obtaining nitrates this way. The first way, called the French method, involved mixing manure with wood ash, straw and urine; the mixture would be tended up to a year, then filtered through more ashes and a bit of water. A second process, called the Swiss method, involved placing a sandpit directly under a stable; only the urine made it into the sand, which would be harvested and filtered in the same manner as the French method. The main disadvantage of this method is that you'll need to process very large amounts of manure to obtain useful amounts of nitrates.
Cody's Lab made a video of a similar method.[1]
A less dirty method involves ionizing air using electric arches, which produces nitrogen dioxide.[2] This is absorbed in alkaline solutions, to yield nitrates.
Making nitrates is more intensive than simply buying them from store. However in some places nitrates tend to be restricted or hard to come by, which makes these method attractive.