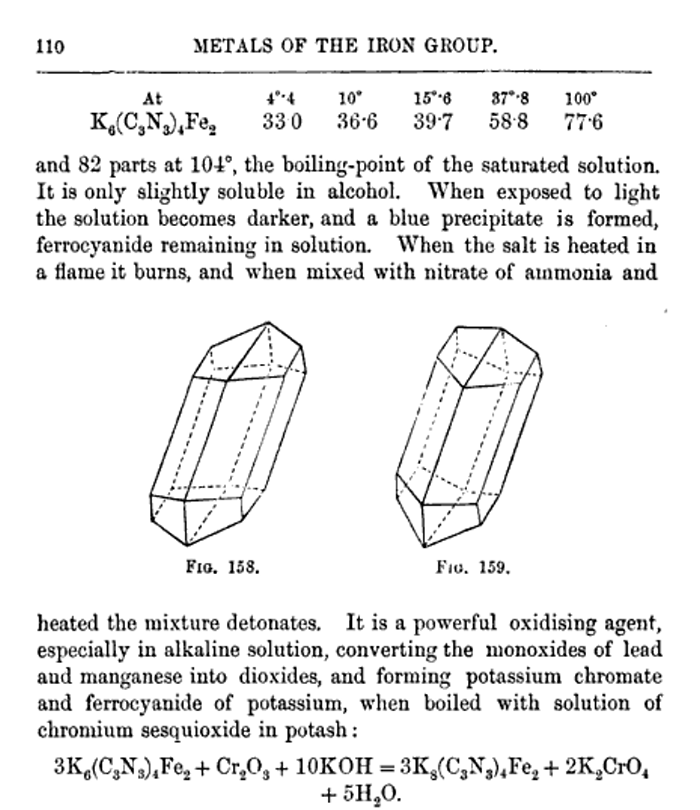
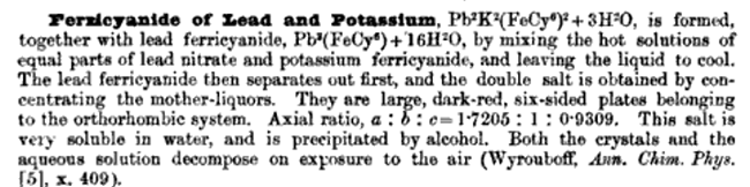"Detonating Composition To Replace Fulminate Of Mercury.— This composition is the invention of Herr J. Fuhrer of Vienna, and it is intended for use
in place of fulminate of mercury for producing the initial detonation necessary for firing a charge of high explosive. It is very much safer to handle
than fulminate of mercury and the inventor claims it will satisfactorily detonate the high explosives. If so, the gain will be very great. One of the
great dangers in handling high explosives has been the sensitiveness of the fulminate fuze or detonator, and no satisfactory detonator not containing
fulminate of mercury has (unless this proves to be the long sought article) been obtained. The materials used are copper, ammonium nitrate,
potassium nitrate, sulphur and aluminum. One formula specifies 30 to 40 parts of copper-ammonium nitrate, 42 to 25 parts of nitrate of potassium, 10
to 7 parts of sulphur, and 18 to 28 parts of metallic aluminum. The date of acceptance of the British patent (No. 20,755) is December 31,
1901." (Proceedings of the United States Naval Institute, Volume XXVIII, Part 1, page 145.)
|