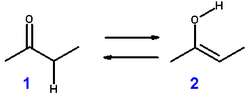stygian - 22-4-2006 at 13:37
If you have a ketone like MEK, would the enol form be:
CH3C(OH)CHCH3 or CH2C(OH)CH2CH3 ?
MassConfusionSquared - 22-4-2006 at 13:57
Both forms would theoretically exists but the more highly substituted carbon would be more acidic, giving up it's proton for a pair of electrons.
H3C-(C-OH)=CH-CH3