MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Dichloromethane Distillation/Azeotropes
DCM is extremely expensive. Even more so when you must order it on the internet (the cheapest I have found is 11 dollars a liter plus shipping or
about $19/L). Because of this I decided to do some OTC browsing today.
I found a product (I forgot the name so I can't check the MSDS but I did remember the ingredients) that claims to contain: Methylene chloride,
isopropanol, ammonia and less than 4% MeOH.
A quick search turned up DCM forming a 98.5% azeotrope boiling at 38.1C with water (I believe). I know it forms an azeotrope with methanol but I
can't seem to find the data. The ammonia shouldn't be a problem as it could just be boiled out or reacted with acid to create an ammonium salt. Does
it form an azeotrope with 2-propanol? What about ternaries?
Is there a good way to seperate secondary and primary alcohols from DCM? Does DCM react with sulfuric acid? If not, could H2SO4 be used to dehydrate
the alcohols to ethers and alkenes? Diisopropyl ether seems like it could be seperated with a good column and dimethyl ether is a gas at RT so it
shouldn't be a problem.
I could have sworn this had been discussed but I can't seem to find it. And my references are temp. unavailable. I'm really not being lazy guys, I
swear.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
http://sciencemadness.org/talk/viewthread.php?tid=1083&p...
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by MagicJigPipe
...
Is there a good way to seperate secondary and primary alcohols from DCM? Does DCM react with sulfuric acid? ... |
After a preliminary distillation to get a DMC-rich fraction, shake that with CaCl2 and filter. The lower alcohols form complexes with CaCl2, which
will also grab any water in the mixture. A second distillation should give a fairly narrow boiling range around 39-40 C.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
some azeotropes
Filter the CaCl2 before redistilling. If it is in a gel form as they usually are, absolutely do not fill the flask more than half full, it will
really have a tendency to bump because of the gelling agent. If you are pushing too hard, and the gel is about to climb the column, take the flask
out of the water/sand bath/heating mantle and swirl it to settle the bubbles.
It may be worthwhile to pop the flask in the refrigerator for a while before treatment with CaCl2.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Why don't you simply wash the alcohols off with water? Then dry and distill.
Damn, i pay 9E for 5L of >98% DCM......
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
DCM is definitely not hard to acquire OTC, however out of interest to keeping these sources secure, I will only say that a proper search will find you
what you are looking for.
That said, it's easy to purify by washing with some water, acid, base, brine a few times, and that's it. It's a bit of work but there's not much you
won't get out that way. Distill if you need to...
Or just buy it from a supplier.
As far as azeotropes are concerned, I translated some info out of a book... don't know how helpful it will be but here you go:
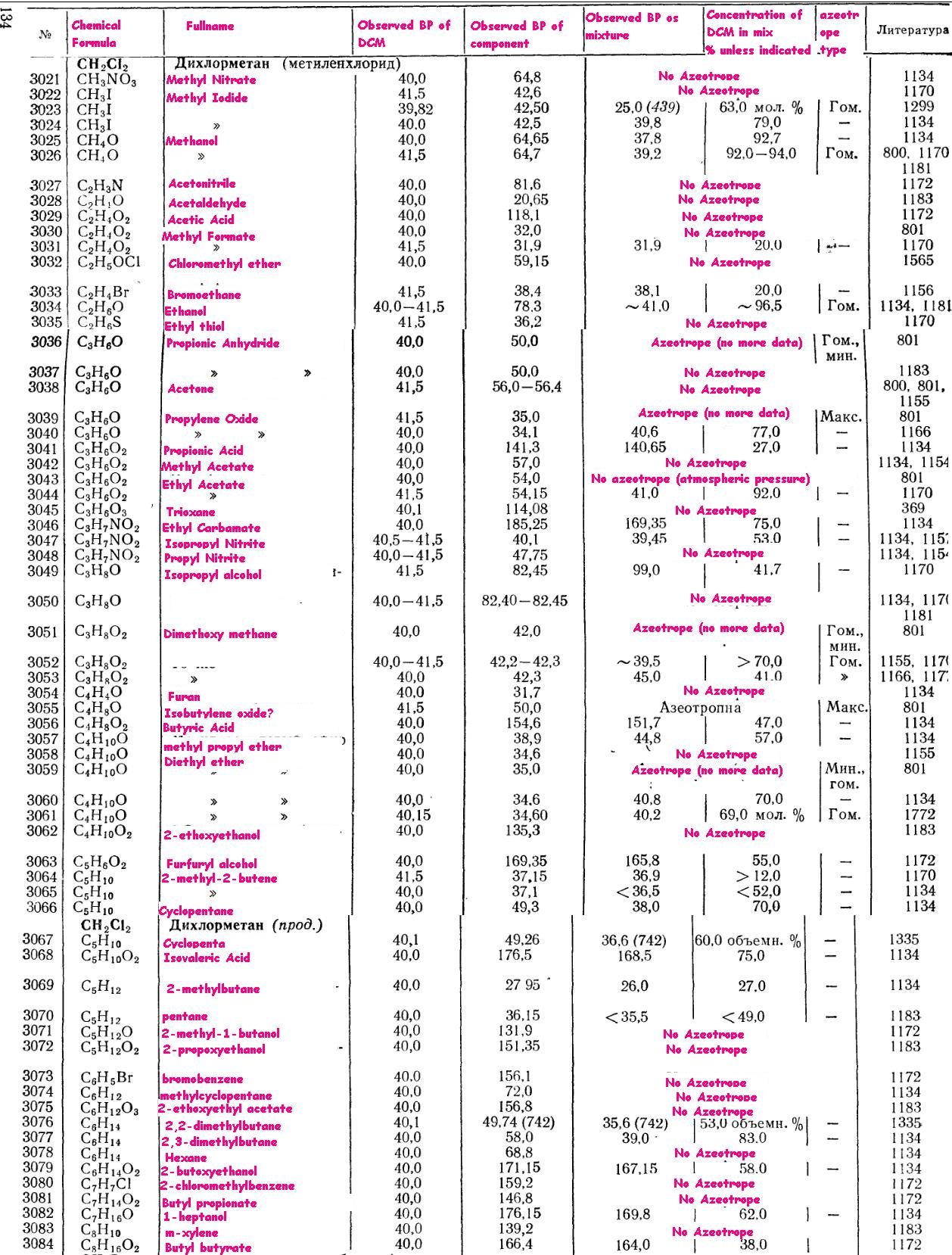
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I've searched pretty good. There don't seem to be any local sources that I can find. I called all places in the phone book that had to do with
"plastic" and none of them had DCM.
Does washing with water really work well? Does it usually require a large amount of water. I'm pretty sure my DCM source is a clear liquid because
it's for automotive paints.
Which complexes more alcohols gram for gram, MgSO4 or CaCl2? I have plenty of both.
Thanks for the azeotrope list Painkilla!
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I would suggest washing with water, CaCl2 solution and drying with CaCl2 before distn. This is usually done when removing alcohols from esters. Water
washes do a pretty good job at removing methanol and ethanol.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Hey MJP, you should check out some of the fiberglass suppliers around memphis.
Been meaning to make a run there myself.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Hey, good idea! If the price is right I wouldn't mind a 4 hour round trip to get some DCM. Of course I'd need to collect a 5 gallon bucket of it or
something similar.
I haven't checked fiberglass places here. Damnit! It's too late on a Saturday.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
What you need to produce DCM from paint stripper most easily is a borosilicate glass adapter with a female ground glass joint that fits your
distillation equipment on one end and a wide enough tube which is inserted into a one hole rubber stopper on the other end  One then pours out a liter of the paint stripper and uses a salt water bath to
distill the crude DCM until the temperature rises above 40ºC One then pours out a liter of the paint stripper and uses a salt water bath to
distill the crude DCM until the temperature rises above 40ºC  One then removes
the heat and after the metal can cools one adds the liter of paint stripper removed earlier back into the metal can and heats it back up and distills
the rest of the crude DCM One then removes
the heat and after the metal can cools one adds the liter of paint stripper removed earlier back into the metal can and heats it back up and distills
the rest of the crude DCM  One then washes the crude DCM with tap water and
then redistills it after drying it with anhydrous calcium chloride overnight One then washes the crude DCM with tap water and
then redistills it after drying it with anhydrous calcium chloride overnight  One
then has about thee liters of reasonably pure dichloromethane One
then has about thee liters of reasonably pure dichloromethane 
Chemistry is our Covalent Bond
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Do you mean use the can as the still? Not a bad idea considering the gel/polymers left behind.. just need to throw it away 
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
The can should be used as a still as else theres a lot of endless washing up. What he means by the pouring 1l out procedure is that there will be too
much bumping if you use the full can - I think 1/2 full is better than 3/4 - and still you can get some gel carrying over. Its best to use glass wool
plugged lightly inside joint to stop this. H2O removes some but not all CH3OH, complexing with CaCl2 does the rest as well as drying - but I think
you save nothing that way and might as well form the CaCl2 adduct without washing. The CaCl2 needs decanting before 2nd distillation, else it will
release some of its bound water.
[Edited on 6-4-2008 by len1]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I'm going with Len here, skip the water wash. DMC dissolves a bit in water, 13 g/l or so, the alcohols will increase the solubility. Just distill the
crude DMC out, complex with CaCl2, filter that out, repeat with a bit of CaCl2 to insure you've got it all, fractionate the filtered DMC. You can
recover the alcohol from the CaCl2 by heating (I've done it in a resin kettle which makes removal of the CaCl2 easy) or by dissolving the complex in
water then distilling the alcohol off (may be better if you just have ordinary flasks).
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Has anyone here concentrated nitric with DCM? I've heard of it and read the patent on it and it looks good, just wondering if anyone has actually done
it. It should be able to be replaced by chloroform too, just less effective. I am also interested in the possibility of nitrating things (glycerin,
toluene, etc.) directly in the DCM. The patent mentions that it can nitrate toluene, but says nothing more on that. What do you think?
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I always thought you could just nitrate glycerin with mixed acids and then just seperate with a separatory funnel. Why would you want to use DCM?
Also, IMO, distillation of nitric acid would be much easier than having to worry about getting an electrode that is unreactive towards it.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Electrode? I'm not sure what you're talking about.
I'm talking about taking something like ammonium nitrate, adding sulfuric, mixing with DCM, then decanting the DCM that has absorbed about 10 or 15%
its weight in pure anhydrous nitric acid, excluding all the salts and sulfuric. You could then simply evaporate it off and get the pure nitric, or as
I was thinking, use it as is.
I think the advantages would be: absolutely no chance of detonation, less chance of a runaway because the DCM would probably boil and cool it before
that happened. Also there would be much less contamination with various other chemicals, the DCM/NG could be decanted, neutralized and distilled at
low temp to leave you with very pure nitroglycerin. Yes it would be a little extra work, but if it keeps my hand from getting blown off, I'm all for
it.
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
The posted procedure is based on JACS 48 562 (1926) which has been a standard method of purification for dichloromethane for
many years  One doesn't apply much heat when distilling a 3/4 full can, then one
has no problem with bumping One doesn't apply much heat when distilling a 3/4 full can, then one
has no problem with bumping  The chart posted above states that there is an
azeotrope of dichloromethane and methanol so a water wash is needed to completely get rid of the methanol The chart posted above states that there is an
azeotrope of dichloromethane and methanol so a water wash is needed to completely get rid of the methanol 
Chemistry is our Covalent Bond
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
In 1926 they weren't concerned with pouring halocarbons into the sewer system, often weren't too concerned with wasting a few percent of a then cheap
solvent. A lot of those older methods are useful but require updating.
And while there is an azeotrope, CaCl2 removes the alcohol too.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
What is DCM used for in the fiberglass "industry"?
497, I could have sworn that I saw something about electrolysis in your post. Maybe that was another thread. Sorry.
Now that I think about it. The DCM/Nitric does sound interesting.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Fiberglass outlets often sell DMC as a solvent, useful in cleaning up.
There was a thread that had a good deal on DMC+HNO3, however I failed at trying to find it quickly.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I called around to some fiberglass "outlets" today. After the first 3 or so had no idea what I was talking about I just got aggravated (and pissed
off at the situation) and gave up. This is rediculous. DCM then NH4NO3... What's next? My guess is sulfuric acid OTC. And H2SO4 any other way is
basically pointless because of the HAZMAT fees.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Products containing ca 90% methylene chloride are readily available in the united states. A brand called klean strip has a lot of products containing
methylene chloride. Go to walmart, look at the strippers they carry, and then cross refrence their products w/ the msds. I have seen a certain
product made by this company everywhere from home depot, to walmart, to lowes, to ace/truevalue, I guarantee if you actually go out and look you will
find it.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Yes, I know there are products that contain methylene chloride. The reason I was looking was to obtain a 100% DCM product so I would only have to
distill once to purify it.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
HA!
Found me some DCM at Advanced Plastics in Nashville (they got several branches all over the southeast US).
Got 5 gallons of DCM for $68 plus some change... the shipping alone would have probably ran at least that much.
Will Calls are the way to go when it comes to chems!
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|