Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
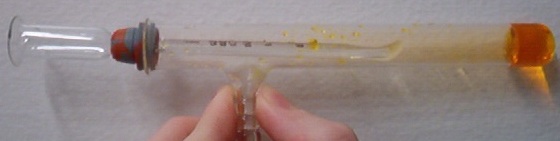
Safe bromine Preparation
I've found a great safe way to create bromine solution. If you mix acetic acid with NaOCl it will generate HOCl which will liberate broming from
bromide salts. It works even better then just bleach
[Edited on 9-10-2008 by Trifluoroacetic]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I have seen a bromination where bromine is made in situ from NH4Br and H2O2 in acetic acid. This might be worth looking into if you want bromine free
from interhalogens.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
I think I read something about that once. I may have to try it.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Isn't it probably best to produce Br2 via Cl2 in a cold concentrated solution of a bromide salt?
I have a hard time seeing why you'd want to do it any other way.
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by kclo4
Isn't it probably best to produce Br2 via Cl2 in a cold concentrated solution of a bromide salt?
I have a hard time seeing why you'd want to do it any other way. |
That is one way of doing it but I live in an apartment and do not wish to produce chlorine gas in order to make a small amount of Br2. I have made it
from sulphuric acid before but I don't keep any really dangerous chemicals or strong acids on hand anymore. An apartment is a bad place to practice
chemistry these days.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
| Quote: | isn't it probably best to produce Br2 via Cl2 in a cold concentrated solution of a bromide salt?
I have a hard time seeing why you'd want to do it any other way. |
Maybe if you are opening a pilot plant; on a preparative scale though, this is really a bad way. There are a few reasons, first chlorine is annoying
to work with and is generally made in amateur settings by oxidizing HCl with something. Next the Cl2 produced goes and oxidizes HBr to get bromine
along with BrCl. This is a little circuitous isn't it? oxidize HCl with something and then use that product to oxidize HBr; additionally the BrCl
which is co-produced makes this Br2 totally useless for most organic preparations.
The only reason why this is done industrially, is because the usual feedstock is sea water, of course for this bubbling Cl2 through is very nice
because it allows an easy way of isolating bromine from a complex soup.
The practical synthesis of Bromine has been discussed on another thread with multiple users posting their lab results, HBr generated in situ then
oxidized with H2O2 seems to be the way to go, other than the bromate preps which look convenient if you have a cheep source.
[Edited on 10-10-2008 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I would of thought NaClO and acetic acid produce Cl2 not HClO.
I would of thought making Br2 form cold sat. (sodium) bromide would be slightly problematic...
First you would need to keep bubbling Cl2 though and not al the Cl2 would dissolve so you would need a decent scrubber...
Secondly the solubility of Br2 in water is larger when Br- salts are in soloution so you would need to fully convert all the Br salt to Br2 by
bubbling exess Cl2 through.
Lastly wouldnt exess Cl2 form a interhalogen with Br2? just a thoguht.
You can generate HClO from conc sulphuric + TCCA.
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
It doesn't matter if you form the interhalogens really as when you come to purify the bromine you would dry it with H2SO4 then distil it from a small
quantity of KBr. The KBr serves to react with the interhalogens and decompose them, liberating bromine and the potassium halide. This is a common
method of purifying both Br2 and I2 (using KI obviously!). It even destroys pseudohalogens such as ICN. When iodine was prepared from seaweed
obviously CN can come about from protein degradation when the kelp ash was formed and when iodine was liberated it was contaminated with interhalogen
galore, sublimation of the iodine from KI served to remove these. The same applies to any halogen purification.
If you require pure bromine for use in brominations where purity of bromine is important then you would normally dry and redistil the bromine anyway
as a precautionary measure, so you should be prepared to distil Br2. It would be common to use it in a solution of glacial acetic acid or
perchlorinated solvent (CCl4). I am not familiar with the peroxide-acetic acid method as it would introduce water and most of the time you would be
using an organic solvent for a reason... the chemicals you are using do not dissolve in water, or you don't want bromohydrin formation, but it would
be feasible if the water ocntent is not critical or deleterious.
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Picric-A
I would of thought NaClO and acetic acid produce Cl2 not HClO.
I would of thought making Br2 form cold sat. (sodium) bromide would be slightly problematic...
First you would need to keep bubbling Cl2 though and not al the Cl2 would dissolve so you would need a decent scrubber...
Secondly the solubility of Br2 in water is larger when Br- salts are in soloution so you would need to fully convert all the Br salt to Br2 by
bubbling exess Cl2 through.
Lastly wouldnt exess Cl2 form a interhalogen with Br2? just a thoguht.
You can generate HClO from conc sulphuric + TCCA. |
Mixing bleach with vinegar produces HClO in fact I used to have an encyclopedia that stated that. It had an experiment for making magnetite from
steel wool and it used this solution to rapidly oxidize the steel to form rust that precipitates from the solution. The rust was then
filtered,washed and heated to form magnetite.
The vinegar is apparently weak enough that HClO is formed and not chlorine gas.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Hmm, I've precipitated bromine from a cold aqueous solution from NaBr and Cl2 gas. I didn't do much with it after that, but to me It looked like a
good way to bromine since it formed a decent layer of bromine at the bottom. The chlorine wasn't to bad since I had it well ventilated.
|
|
|
2bestyle
Harmless

Posts: 4
Registered: 30-4-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: |
The practical synthesis of Bromine has been discussed on another thread with multiple users posting their lab results, HBr generated in situ then
oxidized with H2O2 seems to be the way to go, other than the bromate preps which look convenient if you have a cheep source. |
That is not a good method for preparing lager amounts of bromine. A better method is to deliver HBr with HCl or H2SO4 and oxidate it with NaClO3. For
3mol bromine you need just 1 mol ClO3-. The disatvantage of this method is, that when you use to much HCl then you get chlorine dioxide. But its
better managable then with H2O2
Sorry for my bad English, correct it when you find mistakes!
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
So are you suggesting passing HBr and HCl gas through an ag. soloution of a chlorate?
I havnt heard of this method... could work i suppose... would be better than using H2O2 for those without acess to such a reagent..
|
|
|
2bestyle
Harmless

Posts: 4
Registered: 30-4-2008
Member Is Offline
Mood: No Mood
|
|
No, that is suboptimal! You furnish ( is this the right word for this?) KBr and the Chlorate in a round bottom flask und connect this with a
destillation bridge where you put a dropping funnel on. In this funnel you fill in the hyrochloric acid. The dropping funnel should have a pipe that
is submerge in the KBr Chlorat dissolution. You need to heat with an oil bath to destillate the Br2. After this apparatus you shold connect a ice
coold cooling trap to condense all of the Br2.
|
|
|