Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
Cl Reduction from arylcyclohexylamine
Apologies if this is a noob question, but could someone point me in the direction on what reduction one might use to remove the chlorine atom from an
arylcyclohexylamine,
for example 2-(2-chlorophenyl)-2-(methylamino)cyclohexanone
[Edited on 16-11-2008 by Aubrey]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Did you mean to include the ketone moity? Is it the case that you wish to reduce the Cl without reducing the ketone? I think you have to use a
protecting group for the ketone and then reduce the Cl with H2 and a catalyst like Rainy Ni. Nicodem does this kind of thing maybe he will weigh in.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
yes, , i basically want to remove only the chlorine atom frmo the phenyl ring.
thanks for the info. 
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
I think zinc dust in acetic acid usually works for this kind of thing.
Maybe Nicodem can offer more sophisticated suggestions.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Yeah .. and how about Fe in HCl?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by sparkgap
Maybe Nicodem can offer more sophisticated suggestions.
|
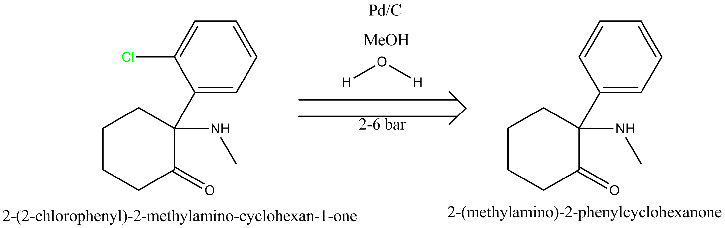
No, I can only offer simpler solutions, like a simple hydrogenation with 5% Pd-C at 2 to 6 bar, though the reduction should be done in H2O/MeOH to
prevent side reactions due to the presence of the amino and carbonyl group. There is plenty literature examples of aromatic dechlorinations, though
I'm not going to search if it was ever done on this specific example since the original poster showed no attempts of actually trying to search the
literature himself. Besides, I don't even know if the substrate is ketamine or is that just an example. Nothing is said that the substrate has an
carbonyl group just like ketamine does. In the absence of the ketone carbonyl group the choice of dechlorination methods is much larger.
Reduction with Zn/AcOH is very unlikely to dechlorinate the aromatic moiety, but it might very likely affect the carbonyl group.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
Thanks Nicodem for your reply.
Apologies for my ignorance, i am just discovering this subject, and the more i read the more fascinated i become.
I've translated your suggestion into diagram form using ChemBioDraw, and attached it (although i can't seem to see it in preview mode).
I am guessing that this avenue is out of my reach if it requires those pressures, since from what i can gather, conventional pressure cookers operate
at only 2 bar. And I would need to bubble H2 through this? Or am i being ignorant here.
I was interested in this technique based on a post on Rhodium about the purpoted increased activity of the compound on the NMDA receptor, i didn't
find any further info than this. And perhaps the whole synth would be required starting with a compound without the Cl atom to begin with (i.e.
benzonitrile)
Found this: http://cat.inist.fr/?aModele=afficheN&cpsidt=17988238.
Catalytic dechlorination of p-chloroanisole (p-chloromethoxybenzene) was carried out in a solution of NaOH in 2-propanol in the presence of Pd/Al2O3
or Pd/C at 40°C.
I wonder if this would work
[Edited on 17-11-2008 by Aubrey]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Maybe look into tributyltin hydride, it is a general reagent for the reduction of halides although I do not know how effectively it reduces aryl
halides. I suspect it should leave the carbonyl alone but am not sure.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Aubrey
I am guessing that this avenue is out of my reach if it requires those pressures, since from what i can gather, conventional pressure cookers operate
at only 2 bar. And I would need to bubble H2 through this? Or am i being ignorant here. |
What? A pressure cooker? Who mentioned that?
When one says some low pressure like 2-6 bars hydrogen atmosphere, a Parr shaker apparatus is implied. So either you buy one or make one. The first
option is quite expensive, while the other is relatively easy except for the reduction valve for hydrogen, which needs to be bought and is expensive
(but at least hydrogen is cheap). Of course, atmospheric pressure can be used, but then you either use 10% Pd-C or higher catalyst loads.
For aromatic dehalogenations with hydrogen usually triethylamine is added as base even though often no base is required at all. Other bases (like
K2CO3, KOH and NaOH) can also be used if soluble in the reaction media. Of course, if the substrate is already a base, then additional base is
obviously not necessary.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
Use a palladium catalyst. I'm not sure which carrier is most suitable but my guess would be a basic alumina. Use a protic solvent and avoid acidic
conditions by adding a base (metal carbonate or hindered amine). Finally, high temp and low pressure promotes hydrodehalogenation.
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
thanks for the info. I wil ldo further research. Nicodem mentions the simplicity of a home made Parr apparatus. I can't seem to find any info on
this, anyone have a link / pdf?
a bit OT but i cracked open a Brita equivalent water filter to see what's inside, and foudn what looks like carbon, and something else. i'm wondering
whether this would be a viable alternative to using activated carbon if i cant buy any... ho hum

|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
No, you really need high quality fine carbon for this, powdering large clumps reduce the specific surface IIRC, and you are never sure what else is in
the carbon.. Even activated carbon for aquarium is said to give mediocre results.. But it would be the best bet if you cannot get real activated
carbon from a supplier.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
one more Question, if i may, mention is made of 'Two other analogs have been found the compound missing the 2-chloro group on the phenyl ring, and
its N-ethyl analog'. I'm imagining what is mean by this is replacing the Cl with ethyl. Would this operation be simpler than the operation discussed
above, i.e. by not requiring a high pressure environent? What is the name of this ethylation process so i can read up about it. thanks in advance for
any help 
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
N-ethyl analogue is the same de-chlorinated compound but with an ethyl group on the nitrogen instead of the methyl...
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
and i guess my next (noob) question would be what substance & process one might use to turn a methylamino group to an ethylamino group. I had a
look through some of my chem books and google , but the answer wasn't immediately obvious 
[Edited on 23-11-2008 by Aubrey]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
There is no direct way of adding a carbon to a secondary N-methyl amine that I know of.
The only way I see right now would starting from the free amine (primary), and N-ethylating it selectively. The substrate could be hindered enough to
allow only mono-ethylation, if not you will need to form the benzaldimine, ethylate it and hydrolyze to the N-ethylamine.
So I don't think you can easily get the N-ethyl analogue from the compound you mentionned.
If I may, I would suggest you try some maybe less complex reactions at first. You seem to have limited knowledge in organic chemistry, and you might
get disappointed by many failures if aiming so high. There are lots of very interesting intermediate reactions you could perform that would able you
to develop some practical experience and thoughen your theoritical knowledge.
Just my advice, don't want you to get sick of chemistry because things never work. Succesfully performing some more basic reactions will surely be
much more rewarding than spedning months of harder reactions at first. You will be much more prepared after a few months training and learning.
Take care.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
I appreciate your response  All of my experience is theoretical so far so I hope
to get my hands dirty soon. All of my experience is theoretical so far so I hope
to get my hands dirty soon. 
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
I have a question about pressure reactions, or rather the last step of the synthesis in the literature of the amine compound from an imine is formed
through a thermal rearrangement using somehting like decalin or ethyl benzoate. Alternatively the use of a pressure bomb is mentioned. From what ive
read on here a pressure bomb is a synonym for pressure reactors / autoclaves, although the two appear to have subty diferent features. (http://www.parrinst.com/). So..
what i'd like to know is a how many atmospheres would this rearrangement require?
and would heating still be required, and which of the myriad of devices would i use (and is this somehting i should purchase),
and with which gas? 
I know thats a lot of questions, but this looks like a step i might actually be capable of achieving 
thanks in advance
[Edited on 27-11-2008 by Aubrey]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Aubrey
I know thats a lot of questions, but this looks like a step i might actually be capable of achieving  |
Those are a lot of questions saying exactly nothing to no one. What rearrangement? On what system? What conditions?
Sorry, but I have doubts you might actually be capable of achieving any such reaction of which you know nothing. Rather listen to the advices given
above and start with some basic experiments - unless of course you have some perverted suicidal tendencies. In any case you will first have to learn
the theory and practical aspects by reading the literature, because from your replies it is too obvious that you know no more about chemistry than
what is thought till the high school level. Good luck.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
sob.. that's ruined my night now.. no leads at all
|
|
|