dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
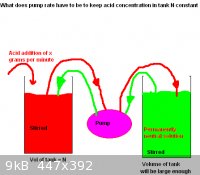
Acid pumping problem
Hello Folks,
Wondering can someone give me a legup with this one. Mathematical modeling is a subject that I am not too hot on. Close to absolute zero?? I was
looking at a model of pollution going into/coming out of a lake but was unable to apply it this problem. It became a ferociously complicated
contraption involving simultanous differential equations involving eigen values for solution etc etc. Way way out of my league.
The system in question is in the diagram below and looks simple enough but it may or may not be simple to model. Can a differential equation(s) (or
any other type of equation) be obtained and solved for to give a figure of what the pump rate needs to be. The pumping will be continous with the
assumption that both solutions are well mixed.
I obtained a model (trial and error really) that will adequately describe the system if discrete pumping is used. That is a pump that is on a timer
and comes on for a short period (say 20 seconds) every five minutes or so. The assumption that must be made with this model is that no mixing of the
solutions can occur during the (short) actual pumping interval, ie. 'pure' acid solution of known concentration is pumped from acidic tank to neutral
tank and 'pure' neutral solution is pumped from the neutral tank to the acidic tank. This assumption can be achieved in practice if pump interval is
short and tubes ends are arranged appropriately and no stirring of contents of tanks is allowed for a short period before pumping (to allow liquid
movement to cease) and during actual pumping.
The model for (discrete) pumped amount is:
Amount = 1 - [[ Total target acid in acidic tank / (Total target acid in acidic tank + Acid added over period) ] X Acidic tank volume]]
To illustrate the formula over a one hour period (any sensible time period can be chosen), assume acid is being generated at a rate of 5 grams per
hour into a two litre acidic tank and our target (& starting) acid concentration is 8 grams per litre. Total acid in the system at the end of the
hour is (8 x 2) + 5 = 21 grams, (10.5 g/l). The required amount to be pumped (neutralized) as per the formula is 0.476 liters. The amount not
neutralized is 2 - 0.476 = 1.524 liters and this amount of solution contains a total acid amount of 1.524 x 10.5 = 16.0 grams of acid which is
distributed in 2 liters giving an acid concentration of 8 g/l which is our target.
The 0.476 liters needs to be pumped fairly quick and may be difficult to do without getting some mixing going on (not wanted) but you could always
take a shorter time interval, say 10 minutes (giving a smaller amount to pump). The example is really only to show the formula working.
The size of the neutral tank is not relevant so long as it is a sensible size and it can be assumed that its supplies neutral solution whenever the
pump want neutral solution (either with discrete pumping or a continous system). Another assumption that can be made is that only liquid of pH 7 will
come accross from neutral tank.
Thanks for looking,
Dann2
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
I think your problem is underspecified. You're not taking into account any sort of buffering effect of the species in solution. The pH is the result
of some set of simultaneous solubility equations. The amount to pump is going to depend on those equations.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Thanks for reply.
I don't think the problem is underspecified as it is a simple acid-tank / neutral-tank system with little or no buffering (or we can assume no
buffering) at all.
The acid being generated is Nitric acid going into a Lead Nitrate solution already containing approx. 6 grams per liter Nitric acid. The neutral
solution contains Lead Nitrate + Lead Carbonate (No actual Carbonate will be going into the Acidic tank by using a filter). This system has (afaik)
no buffering capacity or at least we can assume as a good approximation that there is no buffering going on.
I could state the problem as having two tanks No. 1 and No. 2. Tank No. 1 of size M (1 Litre say if you want to put a figure on it) contains a water
solution of NaCl at Y grams per liter (6g/l if you want to put a figure on it). The other tank contains pure water (zero grams per liter salt).
I add salt (which we will assume immediately dissolves) into tank No. 1 at a rate of X grams per minute (3 grams per minute say if you want to put a
figure on it).
What rate do I need to pump (discard) the salt solution out of Tank No. 1 in order to keep the salt solution at Y g/l (6 g/l say) salt? The volume
of Salt solution pumped out of Tank No.1 is immediately replaced with pure water (from Tank No. 2) of the same volume.
This is the same problem as original acid/neutral problem only using salt/water in the question. What does the pump rate of salt solution out (or pure
water into, same volume anyways) of Tank No. 1 have to be in order to keep the concentration of Salt in Tank No. 1 constant at the starting
concentration (6 g/l if you want to put a figure on it) of salt?
Edited post below:
I figured out the following formula and it appears to work OK as shown by the spread sheet if anyone is interested.
For continous pumping (taking a very short time interval which is so short that you have in effect continous pumping):
Amount to be pumped in delta T = (Acid tank Volume X Acid generated in delta T) / ((Target acid concentration X Acid Tank Volume) + Acid
generated in delta T)
The size of the neutral tank is not relevant so long as it is big enough and able to supply neutral solution at the pump rate. No solids coming over
from neutral tank must also be implemented for formula to be valid.
Edit No. 2
It would appear that you cannot attach .xlr files (Excell spreadsheet).
So I came back and have added a zip!
Dann2
[Edited on 11-5-2009 by dann2]
[Edited on 12-5-2009 by dann2]
Attachment: Accid_out.zip (3kB)
This file has been downloaded 663 times
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by dann2  | | The acid being generated is Nitric acid going into a Lead Nitrate solution already containing approx. 6 grams per liter Nitric acid. The neutral
solution contains Lead Nitrate + Lead Carbonate (No actual Carbonate will be going into the Acidic tank by using a filter). This system has (afaik)
no buffering capacity or at least we can assume as a good approximation that there is no buffering going on. |
An acid and a salt of that acid is the classical buffering situation.
You say the nitric acid is being "generated"; is that by electrolytic reduction of lead nitrate? The way that you posed the original problem is that
nitric acid was being pumped in from the outside, without specifying a concentration.
The other thing that seemed odd about your original problem is that the output of the pump was going into the neutral tank. How would the neutral tank
stay neutral in that case?
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
O Dear, I think perhaps I need to brush up on my pH knowledge but I think that it will not actually effect my particular problem as I am only
concerned with the 'free' Nitric acid in my solution. (Hopeing thats a correct assumption).
The Nitric acid is being generated by electrolysis at an Anode. To show it being pumped in as 100% pure Nitric acid is saying the same thing......I
presume(d).
The neutral tank is going to have an excess of Lead Carbonate, Litharge or Basci Lead Carbonate so that it will always be neutral. I think this is a
fair assumption so long as the tank is sensibly large enough. No solid (Carbonate etc) must come out of the Neutral tank for to keep model simple. A
filter can be used to achieve this.
Going back to the buffering, I added Nitric acid to newly made up solution of Lead Nitrate in water of 350 grams per liter of solution. Small amount
per liter of Nitric acid were added from one gram per liter to approx. 10 grams per liter (100% acid). The values of pH I measured were close enough
to what you get from theoretical measurements of Nitric acid going into water as obtained from this page. I am not saying they were the same (buffering, as you stated is happening no doubt) as the pH meter I was using was not very accurate
at these low pH values but they were close enough to the ballpark accuracy where I am working.
Actually the actual pH value for me is not really relevant. I am more concerned with the actual concentration of 'free' Nitric acid in the solution.
The actual concentration of 'free' Nitric acid in the solution will not be effected by buffering. It will be what it is and will depend simply on the
amount of Nitric acid that you add to a given volume.
I have edited my post above to include the .xlr (Excell) file, wrapped up in a zip, that demonstrates my formula seems to work OK. (for 'free' Nitric
acid anyways, I think). The term 'free' acid is something I always have not been able to understand. What exactly is meant my 'free' Nitric acid.
Sorry about the isolated looking replys. I simply CANNOT get the rquote ............../rquote thing to work for me. We (I anyways) need a long blow by
blow tutorial on how to get it to operate.
Thanks,
Dann2
[Edited on 11-5-2009 by dann2]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I have reloaded the file above is anyone has just downloaded as it had a mistake.
Dann2
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I would take a crack at this problem too but I'm also having trouble understanding it. What are the pumping rates from the two tanks? Your diagram
shows both streams going into/outof the same pump box. Does that mean anything or is it just for drawing convenience?
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
I want to write down the electrolytic half-reactions to make sure we're all talking about the same reaction. I'm assuming that the lead nitrate is
already in solution.
Anode: Pb(2+) + 2 H2O --> PbO2 + 4 H(+) + 2 e(-)
Cathode: 2 H(+) + 2 e(-) --> H2
Combined: Pb(2+) + 2 H2O --> PbO2 + 2 H(+) + H2
After writing down these equations, it became clear to me that the model of "adding nitric acid" is wrong, since you're not actually adding
nitrate ion. The nitrate ion doesn't enter in the reaction at all. What you're doing is converting one charged species, Pb(2+), into another one, 2
H(+), which makes the solution more acidic. Contrast this to the reaction of dissolving litharge in nitric acid.
Ionic: PbO + 2 H(+) + 2 NO3(-) --> Pb(2+) + 2 NO3(-) + H2O
Net: PbO + 2 H(+) --> Pb(2+) + H2O
Litharge acts as a base, albeit one that cannot form a basic solution but can neutralize certain acidic ones (those with, for example, nitrate or
acetate cations).
Now consider a closed-cycle pumping solution as you've proposed. The steady-state solution has the property that the concentration of nitrate must be
the same in both tanks. If otherwise, nitrate would concentrate in one tank and be depleted in the other. Since the electrolysis reaction does not
change the concentration of nitrate, the other tank cannot either. So don't pay attention to the nitrate, since it's not a controllable variable.
The right answer is that the pump rate is directly proportional to the current flowing through the electrolyte, and the proportionality constant is
related the ionic concentrations in the influent and effluent. No matter what solution you pick, it's going to require calibration, because you have
to relate flow rate to electric current.
Whatever the means of calibration, it would be unwise to assume that the Pb(2+) / H(+) ratio in the effluent from the electrolysis tank is the same as
the tank-average. Since these two cations are depleted at different regions of the tank, it's invalid to make a blind assumption that the
concentration is constant. It conceivable, though, that with adequate mechanical mixing that these two ratios become the same.
And one more point. Picking a fixed proportionality between pump rate and current flow is an open-loop control mechanism. It's a good first step, but
it would be useful to figure out what kind of sensor would be cost-effective for closed loop control. It seems that if the flow rate is kept to just
above the Pb(2+) depletion rate, that the effluent from neutralization tank will only ever be slightly acidic and that an ordinary pH sensor would
have long life in such an environment.
[Edited on 12-5-2009 by watson.fawkes]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Thanks Folks,
This is about the tenth time I attempted to post this post. I think I got locked out of the forum (Computational Models and Techniques anyways) as I
posted/deleted/edited way too much in too short a period).
The pump I will be using will be a Peristaltic pump with two hoses going through the pump (a double head each head pumping the same amount). You could
use two pumps instead of course but they would need to pump the same amount otherwise one of the tanks will overflow.
In tank one we have a Lead Dioxide plating under the following equation:
I agree with your equations above Watson.
Assume throughout that we are going to get 100% CE, (in practive we will not get 100% CE and it will continually decrease as plating progresses due to
build up of Nitrites but I am going for a first approximation model here). We get one mole Nitric (63 grams) acid generated for every one mole
electrons (26.802 Ampere hours) that flows.
In the plating tank (the acid tank or tank No. 1) we have the Anode plating (the tank contains Lead Nitrate solution). We also have a desirable Nitric
acid concentration in this tank of approx. 7 grams per liter. 7 grams per liter is actually added to the tank at the start to achieve this desirable
concentration before plateing commences. (I call it the 'target' acid concentration). I wish to keep this concentration constant by pumping the
plating tank (tank No. 1) contents into a neutral tank containing a similar Lead Nitrate solution as the plating tank but the neutral tank (tank No.
2) will have no acid in it as it has an excess of Lead Carbonate (or Litharge or Basic Lead Carbonate) suspended in it. The pump obviously must also
pump the neutral solution back into the plating tank at a similar volume rate as acid solution is pumped into it (tank No.2 ) from tank No. 1.
Once again we assume that no solid Carbonate goes from tank 2 to tank 1 as we use a filter to stop it happening.
What should the pump rate be so that the acid concentration in Tank No. 1 stays at the 'target' acid concentration?
I think myself the formula above seems to work OK. I can give a blow by blow description of how/why I think it is OK as an equation for pumping rate.
Regarding closed loop control using a pH meter is something I will not be doing. I will monitor the process every 6 hours or untill actual needed pump
rate is discovered. (depends on CE and Nitrite build up etc).
Watson.Faskes said:
__________________________________________________________
Whatever the means of calibration, it would be unwise to assume that the Pb(2+) / H(+) ratio in the effluent from the electrolysis tank is the same as
the tank-average. Since these two cations are depleted at different regions of the tank, it's invalid to make a blind assumption that the
concentration is constant. It conceivable, though, that with adequate mechanical mixing that these two ratios become the same.
I am going to assume that both tank contents are homegenous as part of my first approximation as the Pb ions are converted to H ions at a fairly slow
rate and if tanks are being stirred, I believe it will not make a big difference to the model of pump rate needed.
_____________________________________________________________
I think my "equation of pump rate" is OK. Perhaps it's overly complicated but it's just some (very) simple mathematics to get a pump rate value.
Sorry about having to drag the actual application out of me but I though it would only cloud the issue if I had started to dole it out at the start
above. Anyhow it's top secret............
Any more questions please ask.
Thanks,
Dann2
[Edited on 12-5-2009 by dann2]
[Edited on 12-5-2009 by dann2]
[Edited on 12-5-2009 by dann2]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
I (hopefully) have got an answer to my problem. An explanation of my think is given in .gif below. I went over to thinking in terms of H ions as
opposed to 'free Nitric acid'.
There is a spread sheet for checking calculations too.
I got a final formula of pump rate in liters per minute of:
[0.0391762 * Amps into plating tank] / [Tank Volume * Target 'Free Nitric acid' concentration]
Feel free to find any holes in it. Hope it's correct. If not, hope it's not correct, lastly I hope that it will not be described as 'not even
wrong'!!!
Cheers,
Dann2
[Edited on 13-5-2009 by dann2]
Attachment: H_ions_out.zip (18kB)
This file has been downloaded 653 times
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
The tank
volume need not enter into the equation. You can use it, but it's more direct to put (a function of) the target pH in to the equation.
I read your derivation. I recommend learning dimensional analysis and tracking down the units for your constant. As for the derivation itself, you'll get to the answer far quicker by writing
a simple equation of state: the rate of generation of H+ (by the electrolytic cell) is equal to the rate of removal of H+. I'd recommend picking units
of mol/s for these rates.
The first rate is the cell current times the number of moles e- per coulomb times the number of H+ per e- (one). Note that you ever need to do this
for a multiply-charged species, you'll need a different constant here.
The second rate is the molar concentration of H+ in the effluent (in moles-per-liter) times the pumping rate in liters-per-second. The molar
concentration is one-to-one correlated with the pH.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I had another bash at the problem. I think I got it correct this time. I cannot quite figure out how/why the last attempt does not work when I read my
own explanation but wrong it is!
The equation is the same as the last time except the tank volume has disapeared from the bottom of the equation. I worked in Moles H ions per time as
suggested. Think I got my dimensions correct without actually getting demented in the process.
Thanks for input.
Dann2
Attachment: pump_exp.zip (12kB)
This file has been downloaded 630 times
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by dann2  | | I think I got it correct this time. I cannot quite figure out how/why the last attempt does not work when I read my own explanation but wrong it is!
|
It looks right now, although I can't say I read it exhaustively. You've got two constants in there that you
don't explain. I figured them out, but I had to figure them out. Putting their origin down in writing would make the issue clearer, to you as well as
others.
I would recommend spending the time figuring out why your previous attempt was wrong. The difficulty in mathematical modeling is rarely in the
algebra, but rather in constructing concepts that map one's understanding into mathematical objects. So, if you want to really get better at this, go
find the fallacy in your previous (incorrect) derivation. Consider it practice in clear thinking.
|
|
|