DennyDevHE77
Hazard to Others
  
Posts: 149
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Limitations of explosive molecules.
I was thinking about one topic, it is known that the detonation velocity of organic explosives is limited to ~11 km/s.
What about the energy content and density of the explosive? Are there such barriers? As far as I know, researchers pay the most attention to them when
designing a new explosive.
1. Is there a limit to the energy that can be transferred to the molecule?
2. How much energy can the molecule store in principle?
3. Is there a limit to the density?
As I understood, the energy content of the explosive is made up of the oxidation of carbon by oxygen, and the enthalpy of substance formation.
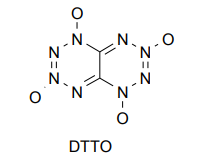
Take octaazocubane (N8), a molecule made entirely of nitrogen. So here all the energy lies in the enthalpy of formation. Calculated at 22.9 MJ/kg. The
question is whether it's possible to cram that kind of energy in by ordinary chemical reactions. Can the initial synthons contain such high energy?
And a question on sensitivity, this molecule, if calculated, contains 5 times more energy than TNT. And because of the absence of carbon and oxygen,
all this energy is contained in enthalpy. Lead azide also has all the energy in enthalpy and is the primary explosive. Wouldn't a potentially
synthesized octaazocuban explode at the slightest whiff of wind?
On the other hand, Philip Eaton, who synthesized octanitrocuban, wrote that this substance could not be detonated by impact, and it has good chemical
and thermal resistance. Of course, it is no longer octaazocubane, but purely geometrically it is also a correct symmetric molecule. Although it is
still pumped with energy and it should be very sensitive or collapse during synthesis. Anyway, I'm kind of confused about it...
And when I think about all this, it seems that such substances (OAC, DTTO, etc) simply cannot be synthesized as there is some conditional limit, but I
have not found information about it.
As for density, as I understood it is achieved by optimal packing of the molecule, but there must be a limit in it too, right?
 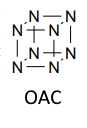
|
|
|
Microtek
National Hazard
   
Posts: 830
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Obviously, there has to be a limit, but AFAIK, the 11 km/s limit is empirically based, and so can't really be taken at face value (since you can't
conclude a negative from empiric evidence). Sensitivity is a matter of activation energy. It may well be that the decomposition pathway is hindered
because of the geometry and electron distribution of a molecule. This can make the molecule much more stable than you would think by looking at the
structure or the enthalpy of formation.
|
|
|
|