ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Will the ester survive?
If someone tried to make an ester out of this and condense the chloride portion of 4-chloro-3-hydroxy-butanal, will the ketone and hydroxide portion
survive? If it does, will the resulting ester survive reductive animation? 
"Imagination is more important than knowledge" ~Einstein
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
What reaction are you proposing? Something is supposed to react with the chlorine in your substrate to form an ester? What reaction is that?
The less you bet, the more you lose when you win.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
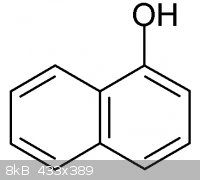
Reacting the chloride portion with the OH part of something like 1-Naphthol. 
"Imagination is more important than knowledge" ~Einstein
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ChemistryGhost  | If someone tried to make an ester out of this and condense the chloride portion of 4-chloro-3-hydroxy-butanal, will the ketone and hydroxide portion
survive? If it does, will the resulting ester survive reductive animation? 
|
What ketone? Do you mean an aldehyde?
What ester? Do you mean an ether?
What reductive "animation"? I assume you mean reductive amination instead, but which method?
If you don't know enough of chemical nomenclature to describe the reaction with words, why don't you simply depict it? It is even simpler than using
words and causes less confusion. Also, be specific. I mean, you ask if something will survive some reaction conditions, yet you don't say what
conditions. There are no general reagents and reaction conditions for the reductive amination of aldehydes (there are dozens of reagents and
innumerable reaction conditions). You should also give a review of the published methods used in this and related syntheses. This would greatly
improve the quality of replies.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|