woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Electrical model of electrolysis cell
I just was wondering how an electrolysis cell behaves electrically. I did some experiments and some research on the physics behind the processes,
occurring at the interface between the electrodes and the liquid.
I have written down all these things in a web-page and I derived a nice and simple electrical model of an electrolysis cell. It is remarkable how
simple that model is, yet, how well it describes the real cell.
Here follows a link of the page. If you have any comments or corrections, please let me know. I'm willing to really understand this subject and
especially if there are errors, I certainly would like to know.
http://woelen.scheikunde.net/science/chem/exps/electrolysis/...
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Hmm so it's exponential minus a voltage drop? Nice to know, thanks. 
That means constant current is definetly the way to go, electrically.
Tim
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Very nice work woelen. I arrived at a current regulated generator for my colloidal metal cell empirically, as after several designs of the power
supply the one that worked best was the one I added an adjustable current function to, where I can now set a voltage and then feed that regulated
voltage into a variable current control. I used a 723 to give voltage tracking and then fed this output into a circuit comprised of a pass transistor
on a heat sink fed by an LM317 wired in a current control method. I find that in playing around with different metals I usually have the voltage set
nearly always the same at the input and spend more time using different currents for various substances. It is nice to see the page you made with the
graphs done so well, your work gives a good reference to look at when I experiment.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nice write-up woelen. I conclude from the general shape of your final cell amperage vs voltage graph that it would be advisable to have a
current regulated power supply for electrochemical work. Is this a fair statement?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
You all have concluded right. Current control is much better than voltage control. I devoted a webpage to this subject as well, for making a power
supply for a small amount of money, which suits almost all electrolysis needs one may have.
http://woelen.scheikunde.net/science/chem/misc/psu.html
This page describes a setup with a rough approximating current control, but without the need of real electronic devices like an LM317. This page is
very simple. This makes the idea also achievable for the chemistry hobbyist, who is a total n00b at the field of electronics. The rough approximation,
presented at the page is acceptable, because electrolysis does not need precisely controlled conditions. Whether a cell is operated at e.g. 1.5 or 1.7
A is not that important, only the approximate value matters.
Also, the setup certainly is not the one with the highest yield in terms of moles of electrolysed material per consumed KWh of power, but for a
hobbyist, who wants to make a few ounces of KClO3 or something like that, that is not an issue at all. For an industrial setup, I would advice a
totally different setup.
I used the setup of this page for electrolysis of NaBr and subsequent adding of KCl to make some KBrO3.
|
|
|
Twospoons
International Hazard
    
Posts: 1282
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
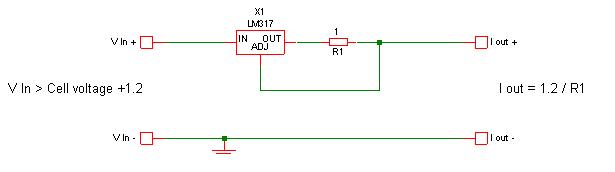
You do realise using an LM317 for current control is insanely simple ...
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
http://www.qsl.net/yo5ofh/hobby%20circuits/power_sourse_circ...
Scroll down to high current regulated supply. I use a circuit like this, with a pot and resistor in series where you see the 75 ohm resistor, so I can
just set current easily. My circuit is a little different but this circuit is great as it is.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Using components like an LM317 is very simple, I know that and I have made a lot of amplifiers, current/voltage controlled sources, etc.  (I have a PhD in electronics and control engineering), but on my site I want things
to be simple, such that n00bs in the field of electronics also can do this. I've seen people messing around with components like the LM317, where they
simply seemed not to understand that pins cannot be exchanged. The result was a LOT of irritation, frustration and not-working things. Yes, even an
LM317 can be blown out if pins are interchanged. Hence, the plain-stupid-simple resistor stuff. At least, these cannot be blown out easily and pins
may be interchanged for resistors (I have a PhD in electronics and control engineering), but on my site I want things
to be simple, such that n00bs in the field of electronics also can do this. I've seen people messing around with components like the LM317, where they
simply seemed not to understand that pins cannot be exchanged. The result was a LOT of irritation, frustration and not-working things. Yes, even an
LM317 can be blown out if pins are interchanged. Hence, the plain-stupid-simple resistor stuff. At least, these cannot be blown out easily and pins
may be interchanged for resistors  . For electrolysis purposes, the approximate
current control, provided by the resistor is sufficient. . For electrolysis purposes, the approximate
current control, provided by the resistor is sufficient.
@Twospoons: In the JPG image you give, Vin should be quite a few volts larger than Vcell+1.2. The LM317 circuitry also needs a few volts for its
operation, so I would go for something like Vin larger than Vcell + 4V or so. On the other hand, you want Vin to be as low as possible, because that
reduces power dissipation in the LM317.
[Edited on 5-5-06 by woelen]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
You could pick an ideal circuit, in my opinion one that includes the option to add a couple 2N3771's, make up a nice silk screened PCB with
instructions, and sell them as a side source of income. You could possibly make some bucks and eliminate the worries about mistakes all while giving
chemists a nice power source for various size cells. Just a thought.
|
|
|
unionised
International Hazard
    
Posts: 5104
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Just a few thoughts-
The temperature rise probably accounts for quite a lot of the observed variation in current with voltage.
The idea that "no current flows until the potential reaches some cutoff voltage" doesn't make sense to me.
The voltage given is usually the standard cell potential for the H2+Cl2 <--> 2 HCl reaction.
Fine, but that voltage is calculated for standard concentrations (ie pressures) of the gases.
Imagine running this reaction the other way ie using it as a fuel cell. Would you still expect to get the full voltage even if there was practically
no H2 or Cl2 because someone had shut the taps to the cylinders?
With a conventional cell chlorine would still be produced even with a rather smaller voltage- it's partial pressuree wouldn't reach 1 atmosphere but,
since the normal concentration of Cl2 in air is practically none, the small quantity that was produced would diffuse away. More Cl- would be oxidised
and a small current would flow.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I think that this can be posted here, since a new thread would not be appropriate:
I have recently made some KBrO3, directly by electrolysis of KBr with a platinum anode and copper wire cathode.
I noticed that the hydrogen evolution at the cathode slowly became smaller and smaller and, after about one third of the calculated running time,
stopped entirely.
The current was still the same and the cell was still hot.
Apparently bromate is easily reduced by a cathode.
The bromate was formed as usual, but also reduced at the same rate, decreasing the efficiency to zero.
Then I remembered that this is also an issue with chlorate cells, but a less important one because chlorate is not so easily reduced. But the current
efficiency of chlorate cells is also impaired by reduction of chlorate at the cathode.
The problem is solved by adding a small amount of Dichromate to the cell liquor. This forms a very thin (invisible) diaphragm of chromium hydroxide
around the cathode and prevents access of chlorate/bromate to it, stopping their reduction.
I dissolved a few crystals of potassium dichromate in a bit of water and added this to my malfunctioning bromate cell.
IMMEDIATELY hydrogen production at the cathode kicked in, every bit as strong as at the beginning.
The cell was left running until no more brown clouds of bromine were observed around the anode and only oxygen was produced there, and the KBrO3
started crystalllizing already during electrolysis even though the temperature was still at 50°C.
Upon cooling, the amount of KBrO3 crystals increased very much and it looked like a spectacular yield.
Moral of the story: Addition of Dichromate is an absolute must with a bromate cell.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | Originally posted by unionised
With a conventional cell chlorine would still be produced even with a rather smaller voltage- it's partial pressuree wouldn't reach 1 atmosphere but,
since the normal concentration of Cl2 in air is practically none, the small quantity that was produced would diffuse away. More Cl- would be oxidised
and a small current would flow. |
I do not understand precisely what you mean. You suggest, that even at a voltage below 2.19 V (redox potential of chloride-->chlorine + redox
potential of water-->hydrogen) still chlorine is formed? Well, I can tell you that this is not true. When a voltage of e.g. 1.5V is applied to such
a cell, then no current flows. You can easily check my statement, by doing the experiment yourself. When you use other electrodes than graphite or
another electrolyte, then of course with 1.5 V or 2 V you can get some current, but with the conditions of my experimental setup, there is no current.
| Quote: | Originally posted by garage chemist
Moral of the story: Addition of Dichromate is an absolute must with a bromate cell.
|
Sorry that I didn't mention this in my previous post, but you are totally right. This phenomenon is perfectly described in the page of Wouter Visser,
to which I refer from my website.
Btw, I started with NaBr instead of KBr. With that, all material remains in solution and it can be separated with KCl.
[Edited on 7-5-06 by woelen]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I made a detailed documentation of the production of KBrO3 with an electrolytic cell, including pictures.
Go to versuchschemie (URL in my signature) and to "Users Schandtaten", where you will find it.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Garage chemist (Stefan on versuchschemie.de???), that is a very nice synthesis, but I have some questions:
My KBrO3 (I made appr. 7 grams several weeks ago in one night of electrolysis at 1 A) is very slightly yellow. Your material is purely white, my
material is off-white.
Now, I am wondering, is this pure white color due to high purity, or is it due to the presence of traces of copper from your cathode, which give a
blue color, which together with the yellow gives an impression of white? I once had a similar phenomenon, making my own CuCl. This stuff looked very
nicely white, but more precise investigation showed that this was due to presence of copper (II), which is cyan under these conditions and with the
yellowish color of other impurities, it made the compound look really brightly white.
The reason I ask this, is my concern about sensitivity to copper of chlorates. I've read somewhere that copper (II) contamination in chlorates is VERY
dangerous. I wonder, is this also true for bromate?
In my little experiment I used two graphite electrodes, treated with some linseed oil. I electrolysed NaBr instead of KBr. This has the advantage that
the very small graphite particles from the anode can settle (this takes a long time!!!), without any separation of solid bromate salt. Then I
carefully took away the liquid, without taking the crap from the bottom (this introduces some loss, but in a long/tall bottle this loss can be
minimized to just a few percent) and then I added a hot concentrated solution of KCl.
I found that KBrO3 is quite dangerous stuff. I mixed some with sulphur powder and then lighted the mix. I can only say: wow, that stuff is really
energetic. I even think that for pyro-applications KBrO3 is too energetic and sensitive and that it is not much more than a nice curiosity for the
home lab (my 7 grams still are present here, I do not have any other nice experiments with KBrO3). One thing, which is interesting though, is that
AgBrO3 is insoluble, so you can easily make that. I do not know whether this is interesting for other applications though.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Yes, I'm Stefan at Versuchschemie. Are you already registered?
I don't think that there was any copper impurity in my material, since I basified the reaction mix and filtered it while hot to remove any copper
hydroxide.
The yellow color in the solution was from the dichromate.
After washing the product with ice water, it was white as snow.
It also contains essentially no bromide, as acidification showed (solution stays colorless).
A copper contamination of chlorate or bromate is only a cause of concern when the chlorate or bromate is used to make pyrotechnic compsitions. Those
are unstable and can self- ignite.
But if you keep your bromate away from combustible materials, copper is absolutely no problem as there is no pyrotechnic mix present which could
ignite, only pure oxidiser.
Another interesting salt is barium bromate, which is also sparingly soluble (0,2g per 100ml).
On addition of H2SO4 to a solution of this, barium sulfate is precipitated and a solution of free bromic acid is obtained.
A curious compound is strontium bromate, which shows the unique behavior of emitting light while crystallizing.
A hot saturated solution of strontium bromate is said to emt little red flashes of light (only visible in the dark) when it cools and deposits
crystals.
[Edited on 8-5-2006 by garage chemist]
|
|
|
unionised
International Hazard
    
Posts: 5104
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"You can easily check my statement, by doing the experiment yourself."
I did, I used roughly the same salt concentration and I got about 7 microamps at 1.5 volt. I didn't have any graphite handy so I used platinum wires.
I replaced these with foils and got 150 microamps at 1 volt. Not a lot but definitely not zero.
Electrode potentials are measured with zero current flow precisely because the intention is to find an equilibrium potential for the reaction. If
there is a current then chemistry is happening at the electrodes, there isn't an equilibrium state and the electrode potentials are not applicable.
The electrode potentials for gas reactions are measured for a gas concentration (ie pressure) of 1 atmosphere (and activity of 1 to be slightly more
precise).
If you drop the gas concentration, the cell voltage falls.
Running the cell as a gas generator (rather than the way that cell potentials are measured) dropping the voltage reduces the equilibrium pressure of
the gas. Since the normal concentration of Cl2 in air is much less than 1 atmosphere Cl2 will be produced at voltages below the "standard" cell
voltage.
I invite you to reinvestigate the low voltage part of the experiment.
(please be aware that, for this experiment, measuring the potential across the supply will give more accurate results because the current drawn by the
voltmeter may be significant - a 1 megohm impedance meter will draw a microamp or two at these voltages. The voltage drop acros the ammeter will be
too small to matter)
[Edited on 8-5-2006 by unionised]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@garage chemist: Nice to see that you have such a white compound without significant copper contamination. I wonder why my material is somewhat
yellow. It is not due to bromide, because on acidification of such a solution it does not become yellow or orange. It also is not due to remains of
dichromate, because on acidification and addition of H2O2, the liquid does not turn blue (very sensitive test for dichromate in oxidizing/acidic
media, due to formation of CrO5). Probably some free bromine is trapped into my solid, although it is a very small amount, because the stuff I have is
completely odourless. Could this faint yellow color also be due to the linseed oil in the graphite rods I used? I can imagine that some of this is
oxidized to some highly colored species. On the other hand, the material I have gives perfectly clear solutions and these solutions also are
colorless, so up to now it did not really disturb me. But, I always thought that bromate was very light yellow, almost white, but now I know that pure
bromate is purely white without any yellow tinge, and suddenly, the somewhat yellowish color of my bromate does disturb me  . Time to make a new batch or recrystallize some of this from distilled water. . Time to make a new batch or recrystallize some of this from distilled water.
I'm reading versuchschemie.de every few weeks or so, and I think it is a superb chemistry site for the German language area. However, I did not
register. I can perfectly read German, and I understand almost 100% of it, but writing is a totally different thing. I do not want to clutter the
forum with my crappy written German language with all the errors with 'der, dem, das, die u.s.w.'  . .
@unionised: Your observations also are quite interesting! My ampere meter has a bad resolution at low currents (it is an el-cheapo device from an
hardware store) and hence I consider everything below 1 mA as zero current  , but I
understand your point. I conducted my experiments from a practical point of view, and I was mainly interested in the high-current area of the
experiment, where currents are in the order of magnitude of several 100's of mA to several A (that is where home-chemists are working for making
chlorates, bromates etc.). But from a theoretical point of view your experiments are quite interesting. What I could do to repeat your experiments is
using a large series resistor (e.g. 100 kOhm) in series with the cell and measure the voltage across that resistor and at the same time measure the
total voltage from the power source. Then I can derive the voltage across the cell. By using different series resistors (e.g. 47K, 22K, 150k, 220k) I
can make up for different currents. My volt meters are quite decent (resolution of 10 mV with an internal resistance of 10 MOhm). I'll repeat the
experiment with this setup, just for curiousity and add that as an addendum to my page. I'll keep you updated. , but I
understand your point. I conducted my experiments from a practical point of view, and I was mainly interested in the high-current area of the
experiment, where currents are in the order of magnitude of several 100's of mA to several A (that is where home-chemists are working for making
chlorates, bromates etc.). But from a theoretical point of view your experiments are quite interesting. What I could do to repeat your experiments is
using a large series resistor (e.g. 100 kOhm) in series with the cell and measure the voltage across that resistor and at the same time measure the
total voltage from the power source. Then I can derive the voltage across the cell. By using different series resistors (e.g. 47K, 22K, 150k, 220k) I
can make up for different currents. My volt meters are quite decent (resolution of 10 mV with an internal resistance of 10 MOhm). I'll repeat the
experiment with this setup, just for curiousity and add that as an addendum to my page. I'll keep you updated.
I still think that the model, I derived, is perfectly suitable for currents from a few mA up to the A range, but the model needs refining at much
lower currents.
[Edited on 8-5-06 by woelen]
|
|
|