evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Molecular sieves... anybody ever used them?
I been using molecular sieves to dry the EtOH I been extractnig from E85. They were purchased off of Ebay a couple years ago, and I decided to try
them out.
I've found that I can regenerate a Kg of sieves and dry a liter or so of 80% EtOH to damn near anhydrous quicker than I can fractionate it to 92% with
my distillation setup.
For regeneration I'm using a cheap tabletop circular convection oven and round cake pan @500ºF for 3 hours. Once an hour I open it up and give the
sieves a nice stir, then at the end of the regeneration process simply funnel them into a jar while still hot. Sieves are actually fairly decent
thermal insulators when dry, and because of that you can go directly from the oven to the storage container without any problems. The only catch is
the convection oven isn't holding up too well at these prolonged temps... the plastic lid housing the top control part has melted and seperated where
the mounting bolts screw in.
Its interesting to note that the sieves work very quickly and get very hot when wet EtOH is added. It also seems as though I'm losing quite
a bit of EtOH every dehydration cycle. In fact I'm starting to wonder if they are 4a instead of 3a like they were supposed to be... they must work,
because my alcoholmeter consistantly wants to stay sunk after draining.
Another thing that I do not particularly care for is the fact that they are 8-12 mesh. That makes them hard to strain, and the EtOH wants to stick to
them causing some mechanical loss. I think in the future I'll start using my E85 seperation water as a rinse to capture more EtOH.
Oh yeah, that brings me to another point, the sieves must be thoroughly washed with water prior to regeneration or else *boom*. Fortunatly I
managed to think of this before I would have learned it the hard way.
I think I'll order another 10lbs or so and start using them on my 15 gallon still.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
Phosphor-ing
Hazard to Others
  
Posts: 244
Registered: 31-5-2006
Location: Deep South, USA
Member Is Offline
Mood: Inquisitive
|
|
I have some 3A and 4A I have successfully used to dehydrate Everclear. Your right about that water rinse before regenerating!
4A holds too much alcohol to be used for this purpose in my opinion.
[Edited on 2-11-2007 by Phosphor-ing]
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
I have 3A and 4A, both get hot when you add them to wet alcohol. 4A adsorbs EtOH and the water, but 3A only water, but it still gets hot. I think mine
are from Aldrich. Heating to 500F seems a bit high to me, as I've heated to 200C with no problems using them.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
This is somewhat off topic but related in applicatiion.
A molecular sieve or dessicant is just a more readily recyclable version of more
common drying agents although more effective except for aluminum amalgam
in the most stringent needs.
Reverse Osmosis used to extract relatively pure water from solutions containing
ionic solutes , most commonly used to produce drinking water by desalinization ,
may possibly be applied in the removal of water from azeotropes such as ethanol.
I have not seen references to this in particular does anyone have knowledge of
this?
An existing hand operated device intended for survival of those shipwrecked at
sea removes about a liter of water per 15 minutes of pumping. It seems to me
that if a recirculated liter of alchohol is pumped through for 5 to ten minutes ,
a significant fraction of any remaining water in the azeotrope could thus be
expelled.

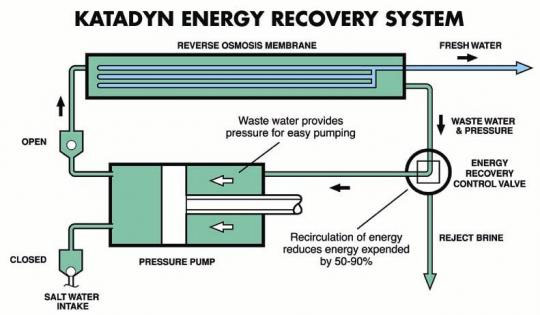
Somewhat pricey but nothing compared to a store bought unit
http://cgi.ebay.com/ebaymotors/PUR-Katadyn-Survivor-35-rever...

He's not kidding these things are expensive.
http://www.amazon.com/Katadyn-8013433-Survivor-35-Desalinato...
http://www.jmsonline.net/KATADYN-SURVIVOR-35-MANUAL-DESALINA...
http://www.basegear.com/desalinator35.html
.
[Edited on 11-3-2007 by Polverone]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Do you think one could use 7a sieves to pull EtOH out of E10? I'm going to try this soon. I'm hoping to use zeolites with appropriate porosity. I
posted about this awhile back.. still thinking about it.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Don't forget that molecular sieves have micro- and meso- porosity as well as nano-porosity. Some of the liquid is held by capillary action, even if
not absorbed into the nano-scale pores. This is why you need to wash or steam treat the used sieves before doing the full reactivation on them, and
why there is some loss of the liquid being dried.
5-A sieves absorb propane and n-butane, 13X sieves absorb many of the smaller alkanes and alkenes up to C6 or higher, including some of the cyclic
ones. This makes the separation of ethanol from petrol somewhat problematic.
There are membranes that will separate ethanol from water, generally they are somewhat hydrophobic in nature and the ethanol diffuses through them.
Commonly they are used in a pervaporation setup, where the ethanol is removed from the other side of the membrane as vapour.
I believe that the common R.O. desalination membranes will let water and alcohol through, while holding back ionic compounds and larger molecules.
When alcoholic beverages have their alcohol content reduced, again both water and alcohol pass through the membrane, and water is added back in to
restore the beverage to its original volume.
Might have a look at the material referenced here : http://www3.interscience.wiley.com/cgi-bin/abstract/10762095...
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
@chemrox:
I do not know where ya got the 7a, but you possibly could get by with 4a. Then again, there are probably a lot of smaller alkanes that they would pick
up as well. And the other problem is regeneration, not to mention it takes a kg of sieves to pull 200g or so or of EtOH.
@franklyn:
There has been quite a bit of testing with RO systems with EtOH. As far as I know, noone has experienced any success.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
chloric1
International Hazard
    
Posts: 1071
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
If one where inclined to use 3A molecular sieves, what would be the best way to use it to dessicate alcohol. Fill a cylindrical column and run fluid
through? Or just add a few ounces to 1 litre of everclear in a beaker? Is stirring helpfull?
Fellow molecular manipulator
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
My sieve is indicating with Cobalt chloride, and I just store my MeOH, EtOH, on up with about 50g of sieve after it's been dried. Usually your bottle
of sieve will tell how much water it can hold, per gram, and you can use this to calculate how much sieve is necessary to remove the water. Figure
that Everclear is azeotropic ethanol...
I would add more sieve to be on the safe side. Look for a duty free shop (i.e. around a military base) and Everclear is pretty cheap. I think it is
about 12 USD/L there.
Edit: The MeOH and EtOH do indeed dissolve some of the indicator and probably need redistilled.
[Edited on 3-11-2007 by Fleaker]
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Just stirring work, flow through column can give a bit drier product if you avoid saturating the last lower bit. Whichever you do, try to restrict
access of air to the alcohol in order to reduce the pick-up of water vapour. Sometimes solvents are stored with some fresh sieves in the container to
help retain the level of dryness, when extreme dryness is needed.
It seems to me that the alcohol would extract CoCl2 to some degree. The indicating sieves that I've seen were intended for drying air or other
reasonably inert gases.
|
|
|