| Pages:
1
2 |
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Aryl Glycols from Aryl Vinyls Oxidation
The standard method of oxidizing a double bond to glycol is aqueous permanganate.
In this instance the double bond is an aryl vinylic one and therefore terminal and conjugated to the aromatic ring.
The specific starting material of interest is 4-vinylpyridine. and the usual permanganate oxidation gives only 35% of the 4-pyridyl-1,2-ethanediol.
The reaction is conducted at 2-4 C and nt allowed to rise above room temp (20 C).
So I am looking for better conditons or a better reagent.
Any suggestions?
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
4-vinylpyridine is of course readily commercially available as stabilized monomer, which ought to be distilled just priot to use.
It was first prepared in 1920 by the reaction of acqueous formaldehyde with 4-methylpyridine in an autoclave, which yields 4-pyridineethanol. This is
dehydrated to 4-vinylpyridine. A lot of tarry byproduct also forms in the first step, and according to a 1947 patent the yield of 4-vinylpyridine can
be increased substantially by treating the crude product and tars rather than isolating the alcohol.
Interestingly that alcohol intermediate can be reduced to the corresponding piperidine and cyclodehydrated (in vapor phase over basic alumina)to
quinuclidine itself. 1-azabicyclo[2.2.2]octane.
The same piperidine-4-ethanol is the byproduct, via hydrogenolysis, of the reduction of pyridine-4-ethane-1,2-diol to the extent of 20%.
[Edited on 17-7-2008 by Sauron]
Attachment: US2556845.pdf (375kB)
This file has been downloaded 557 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
The standard method of oxidizing a double bond to glycol is aqueous permanganate.
In this instance the double bond is an aryl vinylic one and therefore terminal and conjugated to the aromatic ring.
The specific starting material of interest is 4-vinylpyridine. and the usual permanganate oxidation gives only 35% of the 4-pyridyl-1,2-ethanediol.
The reaction is conducted at 2-4 C and nt allowed to rise above room temp (20 C).
So I am looking for better conditons or a better reagent.
Any suggestions? |
Peracetic acid & strong mineral acid should give higher yields.
www.occc.edu/cdodd/documents/chem2115/Unit%205%20-%20Alkenes...
[Edited on 17-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Suppose we rewrite the problem.
Rather than starting with 4-vinylpyridine, we go back to 4-methylpyridine.
Reacting this with formalin produces 4-pyridylethanol, that is, 4-(2-hydroxyethyl)pyridine. This is anyway the intermediate for 4-vinylpyridine.
Now, chlorinating that benzylic carbon would be just as useful as having a hydroxyl there.. If you read the early work by Biet et al, he often used
the 3-chloro compound rather than the 3-hydroxy to form the esters. (I know that does not sound correct but he did. Go read it.)
Adding hypochlorite across the vinyl pi bond is always going to place the Cl in the beta position, while I want it in the alpha position.
So the problem devolves to chlorination of the benzylic position without perturbing the hydroxyl on the 2-carbon.
NCS? TCCA?
Direct chlorination is out because it will attack the activated positions on the pyrudune ring.
A model for this is chlorination of 2-phenylethanol at the 1-position. There ought to be plenty of lit. on this. How to prepare
a-chloro-b-phenylethanol?
[Edited on 18-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
Adding hypochlorite across the vinyl pi bond is always going to place the Cl in the beta position, while I want it in the alpha position.
|
I think this is the better option for a number of reasons.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Ullmann's
Pyridylethanols. Condensation of 2- or 4-methylpyridines with formaldehyde or other aliphatic aldehydes gives the corresponding pyridylethanols due to
the high acidity of the methyl group in the 2- or 4-position
Pyridylethanols are used mainly for the manufacture of vinylpyridines (see Section 3.1. Vinylpyridines).
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Well, back to the glycol.
Here is a quite recent (2006) Org.Syn. procedure that is selective for the benzylic position in chlorination and totally ignores primary alcohols.
Therefore this would yield the 4-pyridyl-1-chloro-2-hydroxyethane that I am after, employing InCl3 (5 mol%) and benzil as catalyst and
chlorodimethylsilane as reagent. See Table 1 for scope of reaction. 2-phenylethanol gives 0% yield.
Whether or not the resulting "chlorohydrin" would survice hydrogenation and cyclodehydration is another question that can only be answered
experimentally - unless it is hiding somewhere in the lit.
[Edited on 18-7-2008 by Sauron]
Attachment: v83p0038.pdf (141kB)
This file has been downloaded 742 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Here are the reaction schemes. The first is the Edgewood patentfor 3-quinuclinol from 4-vinylpyridine. Conversion to the glycol by aq KMnO4 or an
improvement as suggested by Ritter, peracetic acid; conversion to HCl salt (not shown) and catalytic hydrogenation, then cyclodehydration over basic
alumina in vapor phase.
My modification is to chlorinate the glycol alpha-hydroxyl selectively via Org.Syn., then proceed as above to obtain the unscheduled compound
3-chloroquinuclidine.
Ritter says he prefer hypochlorite addition to the vunylic pi bond but that leaves the Cl in the beta position always. Thus far he has not elaborated
how he would cyclize that compound (not shown) to the 3-hydroxyquinuclidine.
My desire is to elaborate a reaction path that explicitly avoids intermediates and product that are CWC scheduled, and which can be used to prepare
quaternized esters of unscheduled glycollic acids potentially useful as smasmolytics and devoid of the delerium-inducing properties of BZ etc.
3-hydroxyquinuclidine is scheduled in Schedule III of CWC and so is benzilic acid. But a number of quaternized esters have been patented and meet the
criteria I desire.
3-chloroquinuclidine can be prepared from the alcohol of course but that means having the alcohol as an intermediate, which is legal quicksand.
[Edited on 18-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
Here are the reaction schemes. The first is the Edgewood patentfor 3-quinuclinol from 4-vinylpyridine. Conversion to the glycol by aq KMnO4 or an
improvement as suggested by Ritter, peracetic acid; conversion to HCl salt (not shown) and catalytic hydrogenation, then cyclodehydration over basic
alumina in vapor phase.
My modification is to chlorinate the glycol alpha-hydroxyl selectively via Org.Syn., then proceed as above to obtain the unscheduled compound
3-chloroquinuclidine.
Ritter says he prefer hypochlorite addition to the vunylic pi bond but that leaves the Cl in the beta position always. Thus far he has not elaborated
how he would cyclize that compound (not shown) to the 3-hydroxyquinuclidine. |
Since this is only a theoretical discussion, my original (and still present) obnjective was to prepare 3-quinuclidinol. The beta-chloro chlorohydrin
is easy to make & would likely cyclize under mild conditions with elimination of HCl. Trying to chlorinate the alpha-carbon & hoping it will
not dehydrochlorinate during the reduction step seems overly complex & problematical.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Well, we already know from the teachings of the posted refernces that the glycol partially undergoes hydrogenolysis in the reduction. It was suggested
therin that the catalyst could be modified to alleviate the difficulty.
I don't see why we have to assume that the situation with the chlorinated alcohol will be any worse (or any better.) I'd assume that a mixture of the
desired 3-chloropiperidine and piperidine itself would form. This side reaction is a nuisance, but only a nuisance, in the patented process, and
likely same in my proposed one.
I did read the DABCO patent so I can see where you were coming from with the 1-hydroxy-2-amine, but the cyclization of the 2-chloro compound does not
appear to be supported by any of the literature so far presented.
I still regard the Edgewood patent as unlikely to ever give better results than the route from ethyl isonicotinate and ethyl bromoacetate. But I
thought I'd leave no stone unturned to find out - particularly if there was a way arund the legal obstancles. This one would accomplish that at least,
but remains low yield. One may be better off preparing the 3-hydroxy compound and converting it immediately to a non-scheduled derivative. That might
suffice.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
The standard prep of 3-chloroquinuclidine is by reaction of 3-quinuclidinol with SOCl2. Your scheme is a) too elaborate, b) too expensive (InCl3???),
and c) likely to fail at the reduction step.
Alkylation of amines can be accomplished using alkyl halides. See http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch22/ch22-3.... I'll dig out some patent examples later when I have the time.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
There are much better reagents than SOCl2, for example cyanuric chloride's complex with DMF, which is very high yielding and rapid at room temperature
in DCM.
SOCl2 is anyway prohibited in this country. Preparing it is an expensive and hazardous procedure employing SO3 and sulfur chlorides. Therefore I
prefer alternatives.
For an example of alkylation of an amine with alkyl halide we need look no further than the ethyl bromoacetate example in the Org.Syn. prep of
3-quinuclidone. That's hardly an enlightenment. My question is directed at a cyclization over hot alumina or aluminum phosphate. Neither the
Edgewood patent nor the DABCO one encomassed a chloride and abstraction of HCl. The options were -OH, -NH2. I doubt the same catalyst and conditions
would avail.
Most likely I can find a selective chlorination less exotic than the 2006 Org.Syn. one. InCl3 was only called for to the tune of 5mol%, or about 13 g
per mole. I haven't seen a price yet, but I suspect it is recoverable and reusable.
Re the glycol, OsO4 is available on solid support, including my favorite PVP. So this reagent may be practical for preparation of the glycol. Use of
su[ported reagent removes a lot of the problems with toxic waste stream.
[Edited on 18-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
For an example of alkylation of an amine with alkyl halide we need look no further than the ethyl bromoacetate example in the Org.Syn. prep of
3-quinuclidone. That's hardly an enlightenment. My question is directed at a cyclization over hot alumina or aluminum phosphate. Neither the
Edgewood patent nor the DABCO one encomassed a chloride and abstraction of HCl. The options were -OH, -NH2. I doubt the same catalyst and conditions
would avail. |
So? Alkylating an amine with an alkyl halide would require its own conditions (basic). I made no statement about either the Edgewood or DABCO
processes working with the chlorohydrin cyclization.
The chlorohydrin would likely be easy & cheap to prepare. I tend to look at the least convoluted chemistry first before pondering Rube Goldberg
reaction schemes that are both expensive & problematical.
| Quote: | | Most likely I can find a selective chlorination less exotic than the 2006 Org.Syn. one. InCl3 was only called for to the tune of 5mol%, or about 13 g
per mole. I haven't seen a price yet, but I suspect it is recoverable and reusable. |
Here is the MSDS for InCl3. Good luck with using it! See [url]http://www.espimetals.com/msds's/indiumchloride.pdf[/url]
| Quote: | Re the glycol, OsO4 is available on solid support, including my favorite PVP. So this reagent may be practical for preparation of the glycol. Use of
su[ported reagent removes a lot of the problems with toxic waste stream.
[Edited on 18-7-2008 by Sauron] |
I prefer the principle of parsimony: the simples answer to a problem is likely the best answer. And your reduction of the pyridine ring will also
remove your alpha chlorine. You may just wind up making quinuclidine (if you're lucky).
[Edited on 18-7-2008 by Ritter]
[Edited on 18-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Ritter
The standard prep of 3-chloroquinuclidine is by reaction of 3-quinuclidinol with SOCl2. Your scheme is a) too elaborate, b) too expensive (InCl3???),
and c) likely to fail at the reduction step.
Alkylation of amines can be accomplished using alkyl halides. See http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch22/ch22-3.... I'll dig out some patent examples later when I have the time.
|
Here it is done in aqueous media (basic) using microwave radiation: http://www.rsc.org/publishing/journals/GC/article.asp?doi=b4...
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Rube Goldberg reaction scheme? Tell that to the editors of Org.Syn.
You're the one who came up with the lame single digit yielding Edgewood patent, not me. An order of magnitude less yield than the route from ethyl
isonicotinate. All I'm doing is trying to get the route you posted up off its knees.
Unfortunately that route is not merely kneeling, it's been amputated just below the waist and has no lega at all.
I negate your criticism by moving the alpha chlorination step to after the hydrogenation. That's still a secondary alcohol, though no longer
benzylic, and is still selectively chlorinated by the method detailed in Org.Syn. by my old friend Rube.
The Edgewood patent is your turkey not mine. It's like cooking a 20 lb bird for Thanksgiving and ending up with only half a wing. All I am trying to
do is to get a decent entree out of it.
Or perhaps the principle of parsimony applies to your holiday meals as well?
[Edited on 19-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
Rube Goldberg reaction scheme? Tell that to the editors of Org.Syn. |
InCl3 is both toxic & expensive. Your benzylic chlorine will pop off as soon as you reduce the pyridine ring. If not Rube Goldberg, then wishful
thinking!
| Quote: | | You're the one who came up with the lame single digit yielding Edgewood patent, not me. An order of magnitude less yield than the route from ethyl
isonicotinate. All I'm doing is trying to get the route you posted up off its knees. |
I only posted that for information, not as an advocate. You stated here that you were not aware of this work.
| Quote: | | Unfortunately that route is not merely kneeling, it's been amputated just below the waist and has no lega at all. |
I guess I made the mistaken assumption that you had decided to swallow your own bile & behave civily. I guess I'm wrong again.
| Quote: | The Edgewood patent is your turkey not mine. It's like cooking a 20 lb bird for Thanksgiving and ending up with only half a wing. All I am trying to
do is to get a decent entree out of it.
Or perhaps the principle of parsimony applies to your holiday meals as well?
[Edited on 19-7-2008 by Sauron] |
See above. I'm finished with your childish behavior.
[Edited on 18-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
The world might condemn it but it's love! 
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I am not married to that Org Syn method, it is just first one I found. If you don't like it, complain to the authors, or the editors of Org.Syn., or
to Wiley. But don't posture about it not being a valid method.
Here's my modified xcheme which removes any and all concerns about lability of Cl in reduction step.
[Edited on 19-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
My modification is to chlorinate the glycol alpha-hydroxyl selectively via Org.Syn., then proceed as above to obtain the unscheduled compound
3-chloroquinuclidine.
|
That procedure is run on an benzylic alcohol adjacent to (and further activated by) an ester function. The environment in the case of the
(4-piperidiniyl)-1,2-ethanediol is entirely different. Can you explain how you intend to regioselectively chlorinate a non-aromatic diol?
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
Rube Goldberg reaction scheme? Tell that to the editors of Org.Syn.
|
That procedure works on a substrate with a single benzylic hydroxyl further activated by an ester group. The chances of it regioselectively
chlorinating your non-aromatic diol are remote. And InCl3 in my Aldrich catalog is $62/gm, which makes the Edgewood cyclodehydration process suddenly
look much more attractive.
And I'm curious as to why your interest has suddenly shifted from anticholinergic CW agents to spasmolytics. Seems odd, that. Anticholingergic
3-quinuclidinol esters can likely be made handily by reacting the sodium salt of a glycolic acid with your 3-chloroquinuclidine.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
On the contrary, the Org.Syn. procedure is run on a wide variety of secondary alcohols as well as benzylic alcohols and is entirely effective at both,
while completely inert to primary alcohols. See Table One in the paper I attached. You must have overlooked it last time around. The reaction is quite
general in scope and highly selective.
I still am looking for other such selective chlorinating reagents.
My interest in CW agents is purely a peper chase. I know too much about such agents to ever want to be anywhere around them juch less prepare them
mysellf. I trust that is clear.
But when it comes to non CWCscheduled, nonmilitary and non-DEA scheduled chemistry, that is entirely a different matter. Outside of those
constraints, I am free to experiment as I see fit. Hence my interest in a route to the chloride that does not proceed via the alcohol.
[Edited on 19-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Wow... And InCl3 in my Aldrich catalog is $62/gm; as I have nearly a kg of InCl3, maybe I can get away without working for the rest of the
year if I can sell it to Sauron. ;-)
Indium recovery and recycling isn't that difficult, people were doing it back when indium was selling at a fraction of current prices. The toxicity
of InCl3 seems to be a bit puffed-up, the studies indicated toxicity with doses on the order of tenths of a gram per kg, which is getting close to
using it as a condiment.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Aldrich is way overpriced particularly on small packaging like 1 g. Likely Alfa is cheaper. Indium III chloride per Merck Index is used in plating
industry. Merck says indium salts are only moderately toxic orally, more so subcutaneous or i.v. Important safety tip. Don't inject any.
1 g InCl3 is enough to run a reaction of the type described on a 75 mmol basis, as it is only required as a 5mol% catalyst. $62 on that basis is not
prohibitive, although clearly this is a reagent that would take a deep pocket for scaleup.
For sure this is not the only chlorination selective for secondary and tertiary alcohols vs primary. I just need to ferret out some others.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
On the contrary, the Org.Syn. procedure is run on a wide variety of secondary alcohols as well as benzylic alcohols and is entirely effective at both,
|
I suggest you read it again. It works with primary, secondary & tertiary alcohols, so there is none of the selectivity you claim. And you still
haven't shown how you are going to regioselectively chlorinate only the secondary alcohol in that piperidinyl glycol.
I've had problems downloading that pdf so here is the reference:
| Quote: | | Organic Syntheses, Vol. 83, p.38 (2006). |
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Really, read it again carefully and check the yields with primary alcohols -0%.
There are 13 examples in Table 1.
Yes it works on tertiary alcohols but there are none such ligands present in the piperidyl glycol, are there?
It does not touch primary alcohols.
Is it expensive? Yes.
Is it practical beyong a bench scale? Probably not.
But it IS selective viz. this glycol.
I just need to go find a similarly selective reagent that is not so exotic and dear.
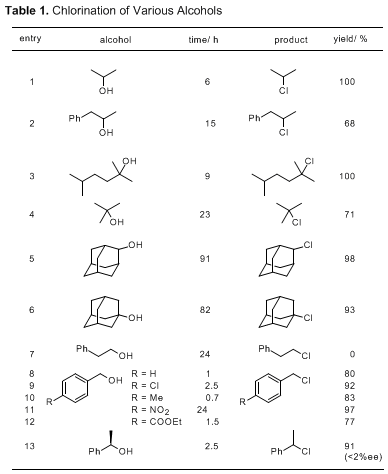
The example of a primary alcohol they used was 2-phenylethanol and 0% yield.
Another example was a compound containing both tertiary and primary hydroxyls and only the tertiary was chlorinated.
Quote: followed by jpg of Table 1
"The generality of this chlorination method is summarized in Table 1. Various secondary and tertiary alcohols are converted into the corresponding
chlorides in high yields (entries 1-6). A primary alcohol (2-phenylethanol) does not give the desired product (entry 7). However, effective
transformation proceeds in the reaction with benzylic alcohols which bear electron-withdrawing or donating substituents (entries 8-10). Nitro and
ester groups tolerate these reaction conditions to furnish the corresponding chlorides (entries 11 and 12). The enantiomerically pure alcohol
(1-phenylethanol) gives racemic 1-phenylethyl chloride (entry 13)."
[Edited on 19-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
| Pages:
1
2 |