Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Very Mild Chlorinating Reagents for Delicate Substrates
In the preparation of amide bonds (peptide synthesis for example) as well as in natural products chemistry there is a need for sophisticated
halogenating reagents that operate under mild, neutral conditions. Acyl halides and alkyl halides are both of interest.
Here we discuss several such reagents:
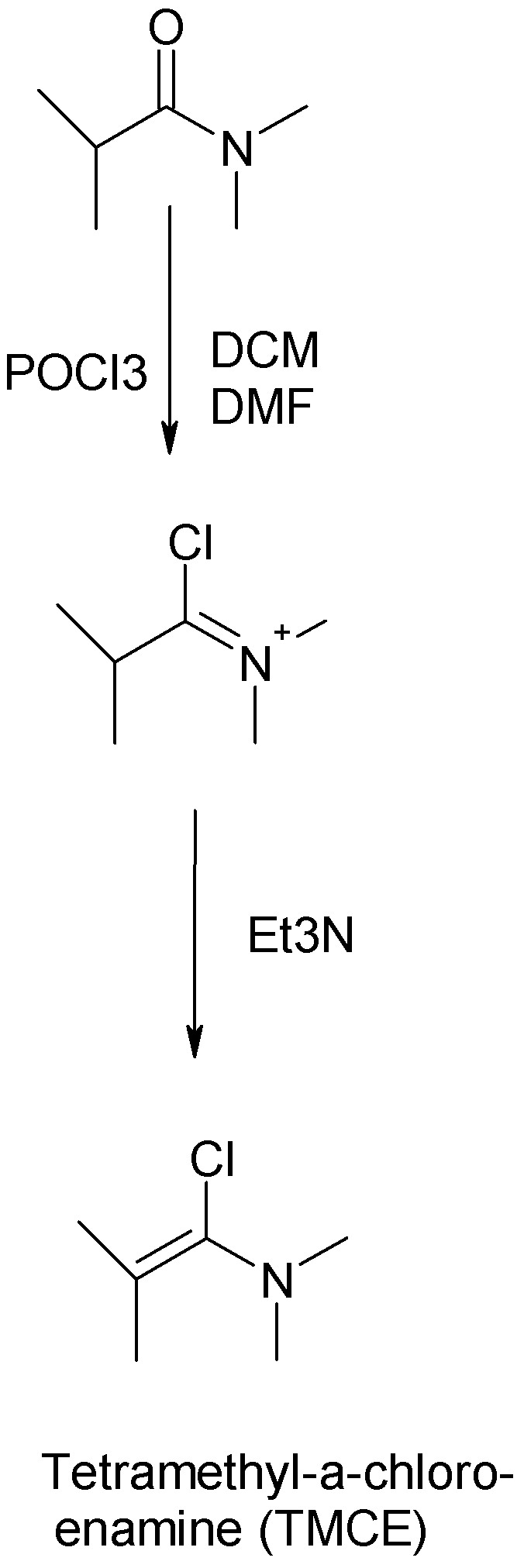
-- Tetramethyl-a-chloroenamine (TMCE)
-- Ph3P-CCl4 and Ph3P-hexachloroacetone
-- CPMA (Chlorophenylthiomethylene)dimethylammonium chloride
Tetramethyl-a-chloroeneamine (TMCE) is a chlorinating reagent for alcohols as well as being useful for preparing acyl chlorides.
The starting material is the N,N-simethylamide of isobutyric acid. Commercially available, you betcha.
Abstract of a Belgian peper in Tetrahedron describing the preparation:
Disubstituted--chloroenamines are useful synthetic intermediates which had earlier been prepared by the reaction of tertiary amides with phosgene. The
toxicity of the latter led us to systematically investigate new synthetic routes towards -chloro- and -bromoenamines. The reactions of various
halogenating agents (SOCl2, diphosgene, triphosgene, OPCl3, OPBr3) with tertiary amides followed by the addition of triethylamine have been studied.
Thionyl chloride was found unsuitable for the preparation of -chloroenamines. Of the other halogenating agents, OPCl3 and OPBr3 were found the most
practical. The generality of the method is illustrated by the synthesis of fifteen -chloroenamines and six -bromoenamines
[Edited on 21-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Here's the paper from Tetrahedron on preparation of TMCE. It is worth noting that POCl3 can be replaced by diphosgene or triphosgene, which are not
CWC scheduled, while POCl3 is Schedule 3. Triphosgene is a solid and readily prepared from dimethyl carbonate by photolytic chlorination. Diphosgene
is a liquid with relatively low volatility and its preparation is described in Org.Syn. I have posted the preparations of both of these reagents
before.
POCl3 gives slightly better (80%) yield compared to 75% for diphosgene and 65% for triphosgene.
See also Org.Syn. 59 p 26 for preparation using phosgene; the synthesis is now superceded but the cotes and references pertaining to the chemistry of
these reagents are now.
[Edited on 21-7-2008 by Sauron]
Attachment: Tetrahedron1998p9207.pdf (976kB)
This file has been downloaded 1555 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
where do you get your N,N-simethylamide of isobutyric acid?
U2U me if you dont want to let it out, thanks 
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Is there any mention of PCL3 or PCL5 been used? Maybe direct chloriantion with Cl2 and a halogen carrier could work also.. Interesting topic.
But could you please do something about those huge reaction schemes? It would be much more readible and pleasant if they were significantly
reduced..
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
There's nn mention of PCl3 or PCl5 in this preparation. A variety of reagents were used succesfully except for SOCl2 which was unsuitable for reasons
given in the article.
From my point of view all of the P-halogen compounds are unobtainable. Red P is unobtainable. POCl3 I can make, but it means bending the law.
Diphosgene and triphosgene I can make legally.
Picric A, try N,N,2-trimethylpropionamide. Or N,N-dimethylisobutyramide. Aldrich 347159. If you can't find it, make it from dimethylamine and
isobutyryl chloride.
Klute old friend, I already reduce the rxn scheme graphic for width to meet forum criteria 700 dpi. I draw them vertically so I can read them.
[Edited on 21-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Other reagents that could be used, instead of PCl3, PCl5, or POCl3, are SiCl4; and AsCl3 although it would be rather more expensive and much more
toxic. In general, to replace a keto group (or an alcoholic or carboxylic -OH group) with an alkenyl chloride group requires a chloride of an element
of which the oxide has a substantially higher enthalpy of formation than the chloride (or oxychloride), as the driving-force for the exchange of =O
(or -OH) for Cl, and does not form a byproduct that would react with the organic chloride. That being the case, I wonder if AlCl3, SnCl4, or TiCl4
could also be used, noting the high enthalpy of formation of Al2O3 or Al(OH)3 and the other oxides.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
John, there is not the slightest indication that those reagents are applicable to the preparation of TCME. In addition AsCl3 is highly toxic and is a
CWC scheduled precursor to Lewisite, therefore unavailable in many places. Read the article. It details half a dozen suitable chlorinating agents, and
one not suitable. Your two candidates are not on the roster. Nor are PCl3 or PCl5.
The older preps were done with phosgene. The reagents tried in the paper posted above were SOCl2 (failure), diphosgene and triphosgene (Success) POCl3
and POBr3 (success.) NO SIMPLE ElEMENTAL CHLORIDES WERE USED.
Meanwhile here is a peper on preparation of alkyl halides using this reagent under neutral conditions.
[Edited on 21-7-2008 by Sauron]
Attachment: TMCE_2.pdf (224kB)
This file has been downloaded 936 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
And here's an example of the original prep using phosgene, from Org.Syn.
[Edited on 21-7-2008 by Sauron]
Attachment: CV6P0282.pdf (241kB)
This file has been downloaded 1296 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
And the use of a derivative of TMCE in the preparation of ketones from carboxylic acids and Grignard reagents.
[Edited on 21-7-2008 by Sauron]
Attachment: CV8P0441.pdf (151kB)
This file has been downloaded 1164 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Triphenylphosphine Systems w/CCl4 or Hexachloroacetone
Another very useful and versatile system for preparing alkyl halides and acyl halides is triphenylphosphine-CCl4 or alternatively
Ph3P-hexachloroacetone.
Here is a Tetrahedron Letters paper on the latter system demonstrating its effectiveness even at -78 C in preparing formyl chloride from formic acid.
[Edited on 21-7-2008 by Sauron]
Attachment: TetLett1997p6489.pdf (261kB)
This file has been downloaded 1136 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
And a review of the Ph3P-CCl4 system in chlorinations, dehydrations and P-N linkage formation:
[Edited on 21-7-2008 by Sauron]
Attachment: Pages from 14.pdf (389kB)
This file has been downloaded 1523 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Also a JACS communication extening this reagent to the preparation of acyl chlorides such as acetyl chloride and propionyl chloride which are of great
interest to many of us.
There's also an example in Org.Syn. of the use of tis reagent in preparing geranyl chloride from the sensitive geraniol.
Org.Syn Collective Volume 6 p 634 (1988)
[Edited on 21-7-2008 by Sauron]
Attachment: ja00966a052.pdf (277kB)
This file has been downloaded 673 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
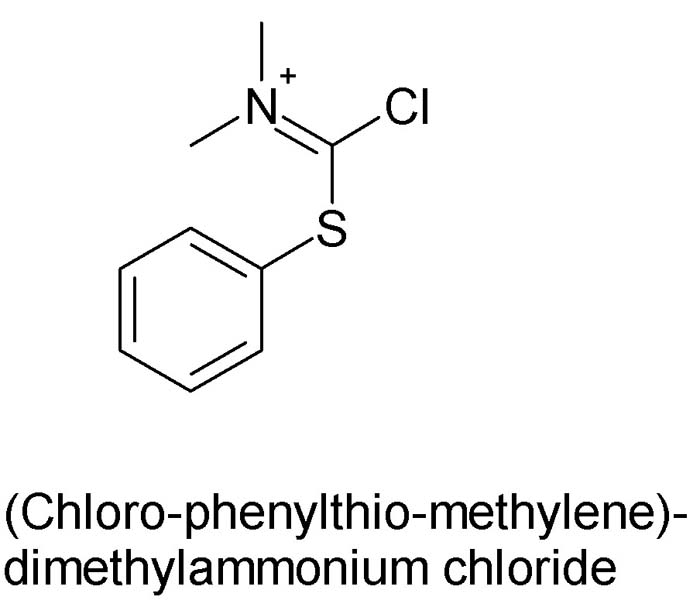
(Chlorophenylthiomethylene)dimethylammonium chloride
To round out the presentation here is another Tetrahedron Letters paper on the preparation and use of CPMA, a mild neutral reagent with that rara
avis, selectivity in chlorination. Only primary alcohols are reactive to this reagent. Secondary and tertiary hydroxyls in same substrate molecule are
untouched.
Note that while the authors used phosgene in this preparation, it can be reasonably anticipated that the usual phosgene substitutes, diphosgene
(trichloromethyl chloroformate and triphosgene, hexachlorodimethyl carbonate can also be used advantageously. Oxalyl chloride is also a very likely
substitute.
Diphosgene is a relatively nonvolatile liquid easily prepared from methyl formate and chlorine. See my threads on this and also Org.Syn.
Triphosgene is even safer to handle, being a solid, and is prepared from dimethyl carbonate and chlorine under UV irradiation in CCl4. I have
previously posted a peper by Eckert from Angew.Chem.Intl.Ed. describing this reagent and its prep.
The starting material for CPMA is S-phenyl-N,N-dimethyldithiocarbamate.
In reference 11 the authors cite the original preparation but also give the following improvement:
Dimethylcarbamoyl chloride (1 g, 8.1 mmol) in DCM 20ml, was added thiophenol (0.67 ml, 6.5 mmol), triethylamine (1.1 ml, 8.1 mmol) and
4-dimethylaminopyridine (10 mg, 0.08 mmol) was refluxed 12 hrs. Basic workup (DCM/sodium bicarbonate aq.) gave an oil. Flash chromatography
(hexane/ethyl acetate 3:1) gave the dithiocarbamate 1.42 g, 89%.
Treatment of the S-phenyl-N,N-dimethyldithiocarbamate with phosgene (3,3 ml 1.93 M in toluene) in toluene 10 mml for 2 hrs 60 C. upon concentration
gave CPMA as a white solid which was washed with toluene. NMR and elemental analysis are reported. M.p. 60 C dec.
Original prep reference:
Copeland and Stick, Aust. J.Chem. 1984, 37, 1483-1487
[Edited on 21-7-2008 by Sauron]
Attachment: TetLett2000p6049.pdf (135kB)
This file has been downloaded 1733 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Apparently the original Aussie prep of CPMA was by reaction of Viehe's salt (N-Dichloromethylene-N,N,-dimethylammonium chloride) with thiophenol.
Full text is posted in next post.
Viehe's salt aka phosgeneimmonium chloride is closely related to the Vilsmeier reagent.
Here is the structure of CPMA:
[
[Edited on 21-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Here's the article from Australian Journal of Chemistry.
I still have to look up the prep of Viehe's salt vs the prep of N,N-dimethylcarbamoyl chloride. This will be the key to whether the French method is
preferable to the Australian one.
[Edited on 21-7-2008 by Sauron]
Attachment: AustJChem.pdf (258kB)
This file has been downloaded 635 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
And a nice ten page review of the chemistry of Viehe's salt written by Viehe himself for Angew.Chem.Int.Ed.
[Edited on 22-7-2008 by Sauron]
Attachment: Pages from 12.pdf (339kB)
This file has been downloaded 974 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thank you for sharing all these very interesting articles.
A one pot reaction to simialr reagentrs could be possible, preparaing diphosgene by chloriantion of methyl formate, and then directly adding the
substarte to the freshly prepared diphosgene.
Obviously, an intial run with isolation of the diphosgene would be needed, but if it works and a adequate solvent for the two steps could be found,
this would make the procedure much more accesable, and limite exposition to diphosgene...
I would love to try a little experiemental work when i get a little more free-time and finish the few incomplete projects. The preparation of
diphosgene would already open the dorrs to many reagents.
Sauron, did you ever try out the chlorination of methyl formate? I remember you discussing it.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Not yet. I did find that the initial chlorination of methyl formate to methyl chloroformate is best done without UV, to avoid risk of explosion. Then
once you have pure methyl chloroformate you can proceed with UV-catalyzed chlorination to the trichloro (perchloro) stage. But you will likely need to
distill the diphosgene, and really all of this screams for a hood.
Triphosgene is just as easy and much safer to handle. It is even commercially available although bloody expensive.
In addition, oxalyl chloride, although nasty, is nowhere near as toxic as diphosgene. The hassle is that it offgasses CO and CO2 in the reaction so
you still need a hood. And it is very irritating and lachrymatory.
Viehe's salt, key starting material for CPMA is best prepared not by the two methods discussed above but according to the review by Viehe, by
chlorination of dithiuram, a cheap vulcanizing agent. A byproduct is SCl2. Thiophenol of course really reeks. Hood again.
Viehe's salt aka phosgeneimonium salt, is very easy to prepare without using phosgene. The commercially available tetramethylthiuram is an inexpensive
vulcanizing agent.
Me2NC(=S)-S-S-C(=S)-NMe2
Chlorination of this initially gives dimethylthiocarbamyl chloride
Me2N-C(=S)Cl 2 mols + 2 S
Continued chlorination gives Viehe's salt and SCl2.
The first stage of this is described in Org.Syn.
Viehe says this is the simplest and best method for preparing his salt.
[Edited on 22-7-2008 by Sauron]
Attachment: CV4P0307.pdf (127kB)
This file has been downloaded 716 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
From this point the story of CPMA fades back into the world of Vilsmeier and Arnold and Viehe chemistry and their antecedents, which is much broader
than just chlorination.
The Vilsmeier reagent is prepared from DMF and oxalyl chloride and dates back to about 1920.
It has oinly one Cl on the methylene.
The Viehe salts are much more recent, and have two Cl on the methylene. Otherwise these are identical, The Viehe salt is commercially available though
expensive.
But as no one seems to be interested I guess I will stand down.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Returning to TMCE and Acyl Halides
Here's a paper from Chemical Communications on the use of TMCE (see upthread) for the preparation of acyl halides (X = F, Cl, Br, I). The yields are
very high, conditions very mild, temperatures ambient or below.
Note that trichloroacetyl chloride is obtained in quantitative yield. This unusual as most methods for preparing this chloride result in partial
decomposition to CCl4 and/or CHCl3. via a radical process. See the H.C.Brown paper on acid chlorides via benzoyl chloride which has been posted
previously.
They also report prep of formyl chloride in 94% yield by same method.
Of the three reagents I have described thus far in this thread, two can be used to prepare both alkyl and acyl halides. Of those two, TMCE is more
versatile and operates at lower temperatures than does PPh3-CCl4, although PPh3 with hexachloroacetone has been demonstrated to operate at low
temperatures as in prep of formyl chloride.
CPMA is the most specialized and the most difficult to prepare but it is also the only one that demonstrates selectivity in chlorination, as it only
reacts with primary alcohols.
[Edited on 23-7-2008 by Sauron]
Attachment: c39790001180.pdf (135kB)
This file has been downloaded 1001 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
For those who, like me, are interested in peptide synthesis, an application of TMCE to complex problems in the total synthesis of cyclopeptides with
cancerostatic proprties is attached. While my own work is nowhere near as intricate, TMCE may well find a place in my synthetic arsenal.
[Edited on 23-7-2008 by Sauron]
Attachment: s-1986-31636.pdf (691kB)
This file has been downloaded 888 times
Sic gorgeamus a los subjectatus nunc.
|
|
|