bbslover
Harmless

Posts: 4
Registered: 1-12-2009
Member Is Offline
Mood: No Mood
|
|
Hi,all, help me draw this structure, ths
-O-CH2CH2-4-pyrrolidin-1-yl-piperidine
I do not known how to connect pyrrolidin with piperidine, also CH2CH2 with pyrrolidin.
How can I identify the number from the cycle? OR is there some web service or software to get this structure directly through inputting the name
like "-O-CH2CH2-4-pyrrolidin-1-yl-piperidine" !
I am beginner. ths
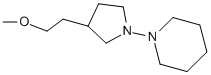
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
The piper-pyrro connection is almost how you have it, but the piperidine's N goes exactly half way around the ring from where you have it.
I'm sorry I can't figure out where to put the ethyl goes. Perhaps it is attached to the N in piperidine. Also, the O should attach to the structure
(if it is indeed an ester), and there should be a double bond between the C=C.
Hopefully someone else can confirm or deny my allegations 
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
You can input (some) names into ChemDraw. What you have is not an IUPAC (International Union of Pure and Applied Chemistry) name, and this chemdraw
won't know what to do with it.
Regardless, keep in mind that simple heterocycles are numbered with the heteroatom as 1. You've also incorrectly called the 3 position of the
pyrrolidine the 4 position. Always chose the lowest numbering when two possible directions can be taken around the ring. From your name, the
attachment of the piperidine ring is ambiguous. You must use parentheses to denote where it is that you're numbering.
The structure you have drawn is 3-(2-methoxyethyl)-1-(1-piperidinyl)pyrrolidine or 1-[3-(2-methoxyethyl)pyrrolidin-1-yl]piperidine. I think the
latter is more 'correct' base on alphabetizing, or perhaps ring size.
[Edited on 2-12-2009 by Arrhenius]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
"-O-CH2CH2-4-pyrrolidin-1-yl-piperidine" indeed is not an acceptable chemical name so nobody can give you an answer (not just that it is
incomplete/ambiguous and against IUPAC rules, but it is not even a name of a compound). The only guess of some certainty that I can make is that
"4-pyrrolidin-1-yl-piperidine" is probably meant to be "4-(pyrrolidin-1-yl)piperidine" because otherwise, just like Arrhenius told you already,
without parentheses, it makes no sense whatsoever. As to what and where the "-O-CH2CH2" part is supposed to be is anybody's guess. When a name is
ambiguous like that, it can fit too many structures and no definite answer can be given so I will not give any. I suggest you to ask whomever made
such a pseudochemical name as he is the only one who knows what he had in mind.
PS: Post beginners questions in the Beginners section.
|
|
|
Nicodem
|
Thread Moved
1-12-2009 at 22:59 |
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arrhenius  | ...
The structure you have drawn is 3-(2-methoxyethyl)-1-(1-piperidinyl)pyrrolidine or 1-[3-(2-methoxyethyl)pyrrolidin-1-yl]piperidine. I think the
latter is more 'correct' base on alphabetizing, or perhaps ring size. |
I thought it could also determined by the most substituted ring, in an attempt to avoid nested brackets as much as possible. As the piperidine ring is
unsubstituted, the pyrrolidine ring would be the base and would have two fairly simple substitutes. Or has that changed since I learned it?
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Hi, maybe you can try a free chemical-structure-drawing-program like ACD labs' one (http://www.acdlabs.com/).
I didn't really understand your question - but you can draw a molecule and it can generate a 'smiles code' and name it also.
One can even have a 3D model of the structure and do various things with it. I've found it useful in the past with visualizations of stereochemistry
etc.
I think one can also enter the cas number etc. and it can draw the molecule for you if that is the problem.
And maybe look at pubchem http://pubchem.ncbi.nlm.nih.gov/
Might be to use if you can find a better name or structure.
Best luck!
[Edited on 2-12-2009 by sonogashira]
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
Quote: Originally posted by Nicodem  | | "-O-CH2CH2-4-pyrrolidin-1-yl-piperidine" indeed is not an acceptable chemical name so nobody can give you an answer |
Oh good, I wasn't going mad then. 
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
bbslover
Harmless

Posts: 4
Registered: 1-12-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | "-O-CH2CH2-4-pyrrolidin-1-yl-piperidine" indeed is not an acceptable chemical name so nobody can give you an answer (not just that it is
incomplete/ambiguous and against IUPAC rules, but it is not even a name of a compound). The only guess of some certainty that I can make is that
"4-pyrrolidin-1-yl-piperidine" is probably meant to be "4-(pyrrolidin-1-yl)piperidine" because otherwise, just like Arrhenius told you already,
without parentheses, it makes no sense whatsoever. As to what and where the "-O-CH2CH2" part is supposed to be is anybody's guess. When a name is
ambiguous like that, it can fit too many structures and no
definite answer can be given so I will not give any. I suggest you to ask whomever made such a pseudochemical name as he is the only one who knows
what he had in mind.
PS: Post beginners questions in the Beginners section. |
HI, thank you all,
I post the original picture now, which is from a paper, and you can give me a picture about the right compound about it.
thank you!
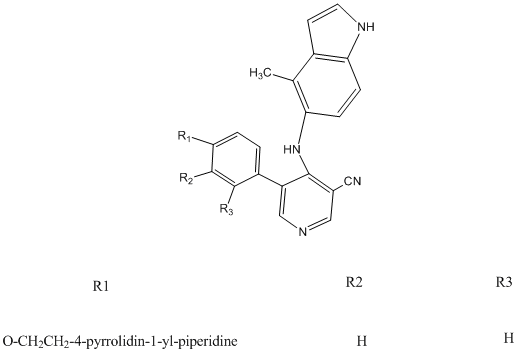
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
In the future, it's helpeful for others if you post the reference in your first post.
Bioorganic & Medicinal Chemistry Letters, Volume 19, Issue 13, 1 July 2009, Pages 3623-3626
Or are you looking at the patent?
BMCL is not exactly a top rate journal, so i'm not surprised by this error. BMCL also generally doesn't publish NMR... so who knows what they actually
made! 
Here's what it probably is.
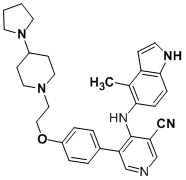
[Edited on 3-12-2009 by Arrhenius]
|
|
|
bbslover
Harmless

Posts: 4
Registered: 1-12-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arrhenius  | In the future, it's helpeful for others if you post the reference in your first post.
Bioorganic & Medicinal Chemistry Letters, Volume 19, Issue 13, 1 July 2009, Pages 3623-3626
Or are you looking at the patent?
BMCL is not exactly a top rate journal, so i'm not surprised by this error. BMCL also generally doesn't publish NMR... so who knows what they actually
made! 
Here's what it probably is.
[Edited on 3-12-2009 by Arrhenius] |
thank you for your help,and suggestion. Next time, I will post my pictures at the first post.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Not just the picture, but please put a reference to the journal article you're asking about.
For example:
Authors, tittle, <i>Bioorg. Med. Chem. Lett. </i>(<b>2009</b> , 19(13), 3623-3626. , 19(13), 3623-3626.
|
|
|
bbslover
Harmless

Posts: 4
Registered: 1-12-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arrhenius  | Not just the picture, but please put a reference to the journal article you're asking about.
For example:
Authors, tittle, <i>Bioorg. Med. Chem. Lett. </i>(<b>2009</b> , 19(13), 3623-3626. , 19(13), 3623-3626. |
yes I get it
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
Don't feel picked on! It's all good information to know 
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|