Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
luminol
I have a very good opportunity to buy some luminol (10g for 10 euro). This is cheap, AFAIK. I'm not yet sure, because i don't have ferrictanide, and
don't want to buy!
Can the same beautiful effect be achieved with other reagents?
|
|
|
woelen
Super Administrator
        
Posts: 8011
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Yes, hypochlorite also gives a nice blue chemiluminiscence.
Why don't you buy K3Fe(CN)6? This is a very interesting chemical, it is not expensive, and not suspicious at all. The same is true for K4Fe(CN)6.3H2O.
To my opinion, any decent home lab should have these chemicals available, at least in small quantity.
If you wish, I can send a small sample of this chemical to you  . .
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by woelen
Yes, hypochlorite also gives a nice blue chemiluminiscence.
Why don't you buy K3Fe(CN)6? This is a very interesting chemical, it is not expensive, and not suspicious at all. The same is true for K4Fe(CN)6.3H2O.
To my opinion, any decent home lab should have these chemicals available, at least in small quantity.
If you wish, I can send a small sample of this chemical to you  . .
|
Does hypochlorite give the same LASTING chemiluminiscence as well?
I might buy ferricyanide. Considering I would be paying the shipping costs alone, that's already to much. From that money I can buy a lot at the
supplier I'm also buying the luminol from.
Already know who i am talking about? Does Malcolm say you something?  
Hes back from vacation.
i will buy some chems, including borohydride, luminol, and some other chems. he virtually has everything for nothing 
If someone in the EU is interested in this supplier, U2U me.
[Edited on 15-1-2009 by Jor]
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
You can use your blood which is free 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
woelen
Super Administrator
        
Posts: 8011
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Luminol does not give a lasting chemiluminiscence at all. In no way can it be compared to the long lasting light of glow sticks (based on oxalic acid
esters of phenols). I never had light output for more than a few tens of seconds. The light is nice though. It has a nice and bright blue/white color.
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
Good point woelen; I was planning sometime to use it for night fishing (to make the floater
visible). Soon enough, I found that it doesn't last long so I switched to something else.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Luminol procedures
Synthesis of Luminol is something I've wanted to do for a long time. I have two procedures, an old one (Brewster, 1961) and a newer one (Pavia,
1998), and then there is a procedure as used in 2007 by Atomisator on the German forum, versuchschemie.de.
1) Precursor 1: Brewster uses 3-nitrophthalic anhydride. Pavia and Atomisator use 3-nitrophthalic acid.
2) Precursor 2: Brewster and Atomisator use hydrazine sulfate. Pavia uses 10% aqueous hydrazine.
3) boiling point elevator: Brewster and Atomisator use glycerol. Pavia uses triethylene glycol (TEG).
I have a large supply of the cheap phthalic anhydride so naturally want to start with this, although I understand it could be easily converted to
phthalic acid. I have no hydrazine and plan on making my own by scaling down the procedure of Mr Anonymous. Glycerol is readily available for me;
TEG is not.
What are the advantages and disadvantages of using the various options I've presented above?
[Edited on 5-5-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I made Luminol a while back.
I started with Phthalic anhydride (much cheaper than the acid) and nitrated this to produce 3-Nitrophthalic acid. This was then boiled with 100%
hydrazine hydrate in glycerol to produce the intermediate. The intermediate was then reduced with sodium dithionite.
Edit- I must note, nitration of the phthalic anhydride produces both 3- and 4- nitroPA's, however when i did this no attempt was made to seperate
them.
the 4-nitro isomer is more soluble than the 3-nitro isomer and so simple washing with water work fairly well but they are both significantly soluble
so a fair amount of product is lost. This does not realy matter and phthalic anhydrde is cheap!
Attachment: 3-Nitrophthalic acid.pdf (138kB)
This file has been downloaded 777 times
Attachment: Luminol synthesis.doc (25kB)
This file has been downloaded 1191 times
[Edited on 5-5-2010 by Picric-A]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks Picric for the benefit of your experience.
It is interesting to note that the complete synthesis of Luminol can also be found on OrgSyn as individual syntheses of each intermediate. They use
phthalic anhydride and hydrazine sulfate for precursors. For the high temperature heating it seems they use tetralin at 160-170C for 3 hours.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Another interesting tidbit discovered on my quest for luminol is that hydrazine sulfate is OTC most everywhere but the US, where its citizens are
being guarded by the FDA. It is sold as Sehydrin, a cancer palliative. But of course, being a "pharmaceutical," its cost is high: like $50/3g.
[Edited on 6-5-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have finally made Luminol, basically from OTC chemicals, except perhaps excluding the phthalic anhydride, which turned out to be phthalic acid. I
converted this to the anhydride as detailed elsewhere on this forum.
I have synthesized hydrazine sulfate (per Mr A) described elsewhere on this forum. This was then converted to hydrazine per Vogel. That is a story
in itself as I had no Cu or Ag RBF. Because I didn't want to break my glass flask, I cut short the distillation and settled for 3% aqueous hydrazine.
An equivalent amount of the 3% worked just fine as a replacement for the 10% hydrazine called for in my Luminol procedure (Pavia et al).
The 3-nitrophthalic acid I synthesized per Littmann and have described this elsewhere on this forum.
The first step of the Luminol procedure is to synthesize 5-nitrophalhydrazide (5-NPH) from hydrazine and 3-nitrophthalic acid. This proceeded
smoothly and a picture of the product is shown below.
Edit2: Taking a lesson from Brewster et al I used glycerol instead of triethylene glycol for the boiling point elevator as I had none of the latter.
The 2nd step is to reduce the 5-NPH to 5-aminophthalhydrazide (Luminol) using dithionite. This also proceeded well with a yield of 93%. Purity is
unknown as the mp is too high for my measuring equipment (320C), but the gold color looked very good. (This is an 18-fold yield over what I obtained
when I conducted this synthesis in school!) See picture below.
And finally, tonight, I made up some chemiluminescent Luminol with the help of KOH and DMSO, as shown in the final picture.
If you have any questions, please ask.
Edit2: Pictures of the apparati used for the 2 steps have been added.
  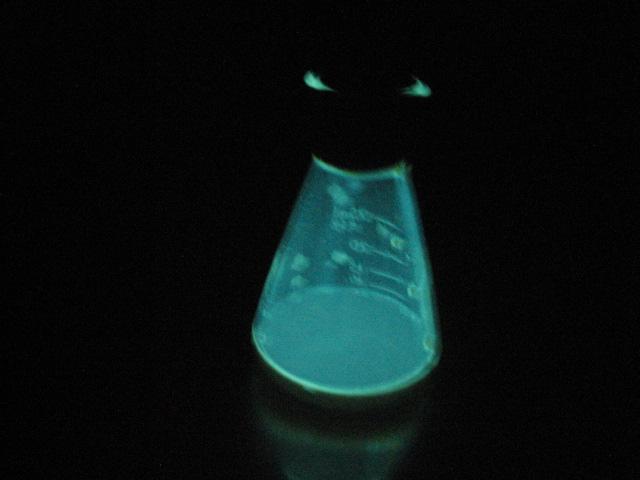
[Edited on 3-8-2010 by Magpie]
 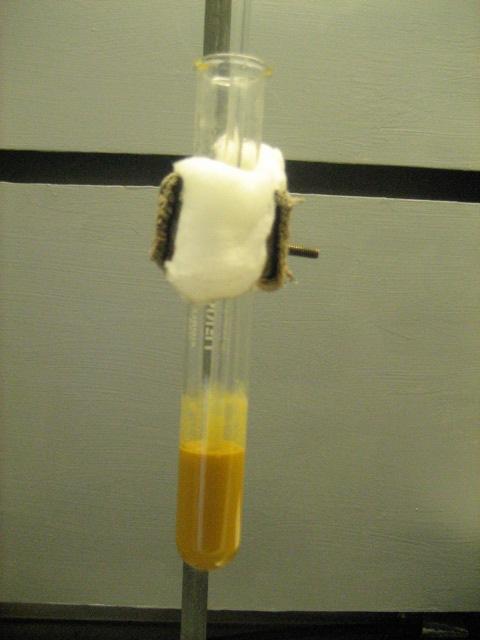
[Edited on 3-8-2010 by Magpie]
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
I was going to make luminol at one time. I synthesized the 3-nitrophthalic acid from phthalic anhydride according to Littman's paper, the yield on a
small scale is fairly low as it appears that this dicarboxylic acid is fairly water soluble. I then broke the flask containing the reaction mixture
containing the precursors for 3-nitrophthalhydrazide while I was heating it in a mantle. All was lost, besides the heating mantle which had to be
heated for a long time to drive out the lost reaction products. Every time that I used this mantle for about 6 months, the residual
3-nitrophthalhydrazide sublimed onto my reaction flask! I am out of phthalic anhydride, so I have not tried this synthesis since then, but I probably
will make another attempt.
Amateur NMR spectroscopist
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by benzylchloride1  | | I synthesized the 3-nitrophthalic acid from phthalic anhydride according to Littman's paper, the yield on a small scale is fairly low as it appears
that this dicarboxylic acid is fairly water soluble. |
Hello BZ1, and thanks for commenting on this synthesis. I was beginning to think that making Luminol was passe.
Yes, I was picking up in my reading that separating the two isomers of nitrophthalic acid was difficult so I decided not to seek perfection here but
just do two washes. Doing this I figure my yield was ~47% with a closed tube mp of 208-218C.
I gave up on getting phthalic anhydride from vendors, but in trying I have now accumulated ~1 kg of phthalic acid. That may last me quite some time
as I work on a small scale. You may have seen my post on converting it to phthalic anhydride. I took my cues from wiki, and your unfortunate
experience with the flask breakage.
http://www.sciencemadness.org/talk/viewthread.php?tid=10156
But do try it again, it's a satisfying experience and the whole family can enjoy the chemiluminescence. They don't get much thrill out of high yields
and good purity.
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
I made luminol as part of the O-chem class in college. I got really good yield - almost the one in the lab manual (82% instead of 86% or something
like that). All the pre-meds clustered around to see the glow.
Chemistry requires (among all the other essentials!) the same kind of care and observation as cooking. If you can't cook, you may be an outstanding
theoretical chemist, but in the lab.....
|
|
|