MitoG
Harmless

Posts: 2
Registered: 30-10-2013
Member Is Offline
Mood: No Mood
|
|
Quinone chemistry and absorption spectrum
Hi everyone!
I hope somebody can help me try and wrap my head around this question that's been bugging me. I haven't studied chemistry in years and, as a
biologist, it's coming back and biting me in the ass.
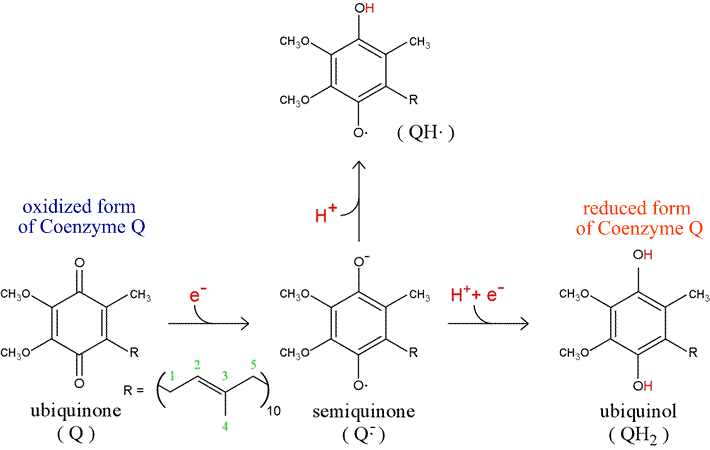
I'm trying to understand what happens to the absorption spectrum when ubiquinone (pictured) undergoes a 2e- reduction to form the reduced form of
ubiquinol (pictured below). There is strong absorbance of the oxidised ubiquinone is at a lamda max of 275nm, however the absorbance drops to near
zero when reduced to ubiquinol. I'm trying to understand what is happening, I've tried reading about conjugated systems and n to pi and pi-pi
transitions to try and explain absorption, but both ubiquinone and ubiquinol look like conjugated systems, so shouldn't they both absorb light
strongly?
Any help would be gratefully received.
Thanks!
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Hi MitoG. You are correct, the reduced 'hydroquinone form' is conjugated, but it is also a special case of conjugation... aromatic.
This results in even greater stability than a conjugated system alone.
You are also correct to believe that this aromatic form is light absorbing... it absorbs very strongly in the UV region. Because it is much more
stable than the conjugated quinone form, it takes higher energy light to bring about excitations, hence while the quinones can get excited by visible
spectrum light, simple aromatics need UV frequencies and so appear colourless.
Hope that helps.
|
|
|
MitoG
Harmless

Posts: 2
Registered: 30-10-2013
Member Is Offline
Mood: No Mood
|
|
Hi deltaH
Thank you for responding! I've been reading around a bit more, and coupled with your explanation - I understand things much more now!
Thanks for your help  ! !
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
FYI, some homemade quinones obviously absorbing in the visible spectrum:
http://www.sciencemadness.org/talk/viewthread.php?tid=23695#...
A further result of quinone chemistry (sorry, I couldn't resist):

[Edited on 1-11-2013 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|