Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
molecular formula and Line formular?
I have a copy of the merk index (cough cough).
I noticed that for Sodium Hydroxide (yes yes my obsession ) it gives the MW as 40.0
But it has too forms of the formula
It has Molecular formula as HNaO
and the Line formula as NaOH
It dosnt give a picture of the structure, so I wanted to know why there is a difference in how the Molecular is written and the Line formula.
If I had to take a wild stab in the dark, I think its to do with how the molecule is actually made up, therefore the formula HNaO represents this.
But why are there two ways to write the same formula?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
There are many ways to use Letters to represent what the Molecules actually look like.
Basically as Knowledge advances, Better ways to represent the Reality are needed, and a simple line of Letters doesn't really work very well.
E.g. Ethanol.
Used to be C2H6O which describes how many of each element is in there.
More recently it is called CH3CH2OH
or even C2H5OH
If you look at the Molecule, as it is understood, these newer ways to write it make more sense, as they better reflect what it actually looks like,
and better identify the bits that are of interest.
|
|
|
kt5000
Hazard to Others
  
Posts: 133
Registered: 27-3-2013
Location: Southwest US
Member Is Offline
Mood: Final exams
|
|
The leading H would indicate the compound is an acid, which it clearly is not. Why would it be written that way?
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by kt5000  |
The leading H would indicate the compound is an acid, which it clearly is not. Why would it be written that way? |
I dont know, I got this from the Merck Index 13th edition. Here is a screen shot. What I dont understand is the difference and the reason why a
compound has two formulas.

|
|
|
kt5000
Hazard to Others
  
Posts: 133
Registered: 27-3-2013
Location: Southwest US
Member Is Offline
Mood: Final exams
|
|
HNaO <-- alphabetical order?
Still seems bizzare to see it that way. I wonder if the other Merck entries are done the same way. No copy of the Merck Index on my shelf 
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by kt5000  | HNaO <-- alphabetical order?
Still seems bizzare to see it that way. I wonder if the other Merck entries are done the same way. No copy of the Merck Index on my shelf  |
I have the electronic version version, u2u me I might be able to get it too you. You need to burn it onto a disk though
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I have checked and looks like there loads written like that.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4320
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
In things like the Merck Index and Chem Abs, they use a specific ordering in the index so that a compound only appears once, and so that the person
looking it up never has to wonder about the correct order- it does not show anything about the compound's properties.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Generally in inorganic chemistry, formulas are written with the least electronegative atom on the right, most electronegative on the left, and
everything between in it's respective place accordingly. However I dislike this method on occasion, and prefer to write them based on their structure
as is done in organic chemistry.
With NaOH this is done anyway - one oxygen bonds to one hydrogen (covalently) and to one sodium (ionicly): Na+~O--H.
Also with NaOCl: Na+~O--Cl or NaO3Cl: Na+~O3--Cl.
It gets quite confusing with bigger polyatomic hydrates like Iron (III) chloride hexahydrate which is commonly written FeCl3⋅6
H2O. This suggests that chlorine oxidizes the iron, <s>which is not the case, chlorine bonds to hydrogen, while oxygen bonds to the
iron.</s> A better way to write it is: [Fe(H2O)4Cl2]Cl⋅2H2O, and a better name is
tetraaquadichloroiron(III) chloride dihydrate, which more clearly represents its structure.
[EDIT, <s>Text</s> is incorrect.]
[Edited on 18-9-2014 by Zyklon-A]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4320
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Zyklon-A  |
It gets quite confusing with bigger polyatomic hydrates like Iron (III) chloride hexahydrate which is commonly written FeCl3⋅6
H2O. This suggests that chlorine oxidizes the iron, which is not the case, chlorine bonds to hydrogen, while oxygen bonds
to the iron. A better way to write it is: [Fe(H2O)4Cl2]Cl⋅2H2O, and a better name is
tetraaquadichloroiron(III) chloride dihydrate, which more clearly represents its structure. |
Bolding mine.
Er, what? You've claimed that the true structure has two chloro ligands on the iron, which is not "bonding to hydrogen". Two chlorines are
covalently bonded to iron; the other is held by electrostatic attractions.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Ok thanks, I didnt know why so thought I would ask 
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
DraconicAcid, Oh I see. I got that formula from Wikipedia, and I guess I interpreted it wrong. Are you saying the formula is wrong?
Or my interpretation?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4320
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Zyklon-A  | | DraconicAcid, Oh I see. I got that formula from Wikipedia, and I guess I interpreted it wrong. Are you saying the formula is wrong?
Or my interpretation? |
Your interpretation is wrong.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
OK, So is this the correct structure:
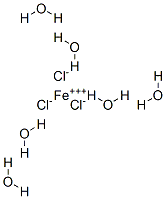
|
|
|
DraconicAcid
International Hazard
    
Posts: 4320
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
No. Assuming the Wiki formula is correct, you'll have an iron with four water molecules bonded to it through the oxygen, and two chlorides bonded to
it (an octahedral arrangement). This will be a complex ion; one chloride will be a counterion, and the two other water molecules will be somewhere in
the crystal structure, but not actually bonded to things.
Similar to this, but with iron.
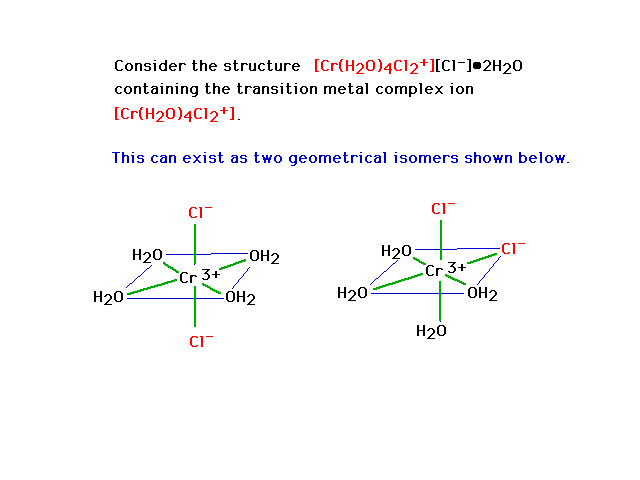
[Edited on 18-9-2014 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|