casasalvia
Harmless

Posts: 2
Registered: 15-1-2019
Member Is Offline
|
|
Question about hydroxylimine hydrochloride
Me and a friend had a debate about hydroxylimine hydrochloride cas number: 90717-16-1
I drew 2D structure of hydroxylimine and I think this is (1-hydroxy-cyclopentyl)-(o-chlorophenyl)-N-methylketimine.
I think this is quite obvious but maybe I'm wrong, we really need confirmation from a third source to end the debate. Anyone who knows about these two
substances please help us, we are very grateful for any help.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
I just looked this up. Not an expert.
1-((2-Chlorophenyl)(methylimino)methyl)cyclopentanol hydrochloride
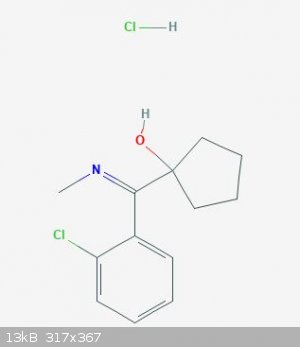
EDIT:
This is broken down, If I am not mistaken. (There might be a mistake, have not touched academic OC in a long while)
(1-hydroxy-cyclopentyl)-(o-chlorophenyl)-N-methylketimine.
Your mistakes I found (and can name  ): ):
1) You start naming with biggest structure. (phenyl ring)
2) You simplify as much as possible (1-hydroxy-cyclopentyl = pentanol) (don't number branches, if there is no difference)
3) Number brunches, when it matters. (phenyl ring has 2 branches, start numbering from largest branch to closest branch)
As I said, not an expert, so that is all I can say. 
[Edited on 15-1-2019 by TheMrbunGee]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
When you say hydroxylimine hydrochloride I would think you mean the hydrochloride salt of formaldoxime. Which apparently forms a stable timer (https://www.sigmaaldrich.com/catalog/product/aldrich/238481).
Your Cas number is much more specific and is indeed the structure posted above. A very suspicious molecule as it, in fact, thermally decomposes into
ketamine at temperatures in excess of 100 C.
Would you care to elaborate on the nature of the 'debate' you had?
[Edited on 15-1-2019 by Sigmatropic]
|
|
|
Tsjerk
International Hazard
    
Posts: 3031
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
1-((2-Chlorophenyl)(methylimino)methyl)cyclopentanol hydrochloride is correct, but the rules TheMrbunGee put down aren't.
Rule 1)
| Quote: | Identification of the parent hydrocarbon chain. This chain must obey the following rules, in order of precedence:
1a) It should have the maximum number of substituents of the suffix functional group. By suffix, it is meant that the parent functional group should
have a suffix, unlike halogen substituents. If more than one functional group is present, the one with highest precedence should be used.
1b) It should have the maximum number of multiple bonds.
1c) It should have the maximum number of single bonds.
1d) It should have the maximum length |
1a) There are only two functional groups here, as for rule 1a halogens don't count; imine and alcohol. both of the substituents are on different
carbon chains, so the maximum number of substituents is 1. Alcohol is higher in precedence, so cyclopentane is the parent carbon chain.
1b, 1c and 1d don't have to be considered anymore.
Rule 2)
| Quote: | | Identification of the parent functional group, if any, with the highest order of precedence |
This is alcohol, as the cyclopentane only has one functional group.
Rule 3)
| Quote: | | Identification of the side-chains. Side chains are the carbon chains that are not in the parent chain, but are branched off from it.
|
This is methyl which has its own branch benzene
Rule 4)
| Quote: | | Identification of the remaining functional groups, if any, and naming them by their ionic prefixes |
These are chloro- and methylimino-
Rule 6)
| Quote: | Numbering of the chain. This is done by first numbering the chain in both directions (left to right and right to left), and then choosing the
numbering which follows these rules, in order of precedence
6a) Has the lowest-numbered locant (or locants) for the suffix functional group. Locants are the numbers on the carbons to which the substituent is
directly attached.
6b)Has the lowest-numbered locants for multiple bonds (The locant of a multiple bond is the number of the adjacent carbon with a lower number).
6c)Has the lowest-numbered locants for prefixes |
Both cyclopentane's side chain and functional group have the same locant, so that is easy; that carbon gets number 1. 6b/6c don't have to be taken in
consideration.
Rule 7)
| Quote: | | Numbering of the various substituents and bonds with their locants. |
In this molecule there is only one branch; methyl. Methyl has both a branch and a functional group; both are on the same locant as methyl only has one
locant. So they are ordered alphabetically, C(hloro) comes before M(ethylimino). The 2 doesn't count for the alphabetic order as for example tri- and
di- also wouldn't count.
Therefor 1-((2-chlorophenyl)(methylimino)methyl)cyclopentanol
chlorophenyl and methylimino are equal groups of methyl which is a group of cyclopentane on position 1 which has the suffix -ol because the highest
precedence (or only) functional group is used as the suffix
https://en.wikipedia.org/wiki/IUPAC_nomenclature_of_organic_...
[Edited on 15-1-2019 by Tsjerk]
|
|
|
Tsjerk
International Hazard
    
Posts: 3031
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Maybe his cat needs surgery?
hydroxylimine isn't quite right indeed, but hey, the guy is asking about nomenclature.
|
|
|
casasalvia
Harmless

Posts: 2
Registered: 15-1-2019
Member Is Offline
|
|
Quote: Originally posted by Sigmatropic  | When you say hydroxylimine hydrochloride I would think you mean the hydrochloride salt of formaldoxime. Which apparently forms a stable timer (https://www.sigmaaldrich.com/catalog/product/aldrich/238481).
Your Cas number is much more specific and is indeed the structure posted above. A very suspicious molecule as it, in fact, thermally decomposes into
ketamine at temperatures in excess of 100 C.
Would you care to elaborate on the nature of the 'debate' you had?
[Edited on 15-1-2019 by Sigmatropic] |
Yes, my argument started when my roommate started trying to prepare ketamine. I asked him why not buy hydroxylimine hydrochloride from China. I
pointed out to my friend that they were the same substance, but my friend said not. Besides, my friend did not trust my chemical knowledge enough to
identify these two substances. If I'm wrong, it's very normal, I'm always willing to accept that. Thank you everyone especially Tsjerk, thanks for
your kind help.
[Edited on 16-1-2019 by casasalvia]
|
|
|
Tsjerk
International Hazard
    
Posts: 3031
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
How can there be discussion about the identity of a compound with a cas number? I don't get it. IUPAC naming, I get. Structure? No
|
|
|