| Pages:
1
2
3
4 |
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
SnailsAttack, I put here a link to a scan from "Palmer, Experimental physical chemistry", the chapter about experiments with solid solibilities. I
hope some practical hints from this book could be useful to your experiments.
https://drive.google.com/file/d/1wzUomQlu6dqQWDhW2UloAqPizGy...
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 165
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Thanks, I looked it over. The procedures for measuring solubility in part A are a little weird, but the titration method is interesting.
In part B the author suggests that only one hydrate can exist in solution at a given temperature, which is what I hoped would happen, since it
simplifies things a lot.
|
|
|
yobbo II
National Hazard
   
Posts: 758
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
SnailsAttack
"24 hours?
I give it five minutes max before a sample of solid copper sulphate emersed in water is effectively fully equilibriated with the solution."
How do you actually know it is equilibriated.
If you are going up the the solubility limit (at a certain temperature) it can take ages to equilibriate.
The next dude may feel he has to give it 4 minutes (or 10 mins. or hours) to be fully equilibriated.
I would imagine that with some substances (not copper suplhate, but I don't really know) it could take weeks!
Sulaiman
"
Help prevent my brain from breaking!
What would happen if anhydrous copper sulphate is added to an already saturated solution?
"
The anhydrous stuff may actually suck some water from the solutions and become a hydrate of some value. It depends on whether or not the system (at
it's temperature and pressure) at that point is congruent or incongruent.
From Wiki:
congruent/Incongruent dissolution
Many substances dissolve congruently (i.e. the composition of the solid and the dissolved solute stoichiometrically match). However, some substances
may dissolve incongruently, whereby the composition of the solute in solution does not match that of the solid.
Trust me.
I am no expert.
Perhaps someone here can put it a better way.
I don't really understand what is meant by '(i.e. the composition of the solid and the dissolved solute stoichiometrically match)'
A phase diagram can explain a system (assuming you can read/interpret) well.
One has been produced and may appear over in Ref's.
Yob
[Edited on 8-3-2023 by yobbo II]
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Quote: Originally posted by Texium  |
I was typing out a longer response last night, but I lost it by accident, and teodor and Amos pretty much covered what I was going to say.
|
Oh, yes, it happend for me several times already. Now I always copy responses to an external text editor when they become long and continue there.
I think there is a nice demo illustrating your and Amos idea "there are a lot of variables some of which are unknown".
https://en.wikipedia.org/wiki/Storm_glass
|
|
|
Sulaiman
International Hazard
    
Posts: 3678
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
You only need to know that it has equilibriated to within your experimental error limits.
A one-time experiment could be used as a reference for 'time required for a given error range'
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Texium
Administrator
       
Posts: 4566
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Sulaiman  |
You only need to know that it has equilibriated to within your experimental error limits.
A one-time experiment could be used as a reference for 'time required for a given error range' |
Not sure
what you mean exactly by “one-time experiment.” Generally if one wants to obtain accurate data, running experiments in triplicate is standard,
especially if you’re trying to establish an error range. Plus, you’d have to control for temperature, particle size of salt that you’re
dissolving, and temperature. As in, making sure that the temperature never gets above a given temperature during the course of the experiment, not
just that the final temperature is the same.
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Did somebody observe the phenomenon when on long standing some saturated solutions of salts in water deposit crystalls and this is impossible to
explain by temperature just because the deposit doesn't disappear during further temperature fluctuations?
The standard method of measuring solubility includes agitation of crystalls and this is also some energy which also participates in the equilibrium
even if we keep temperature constant.
The mechanism of crystallization from saturated solution goes through some step of creating pre-crystal film with different (lower) solution
concentration. So, in normal equilibrium there are always 2 zones with different concentrations of solution - on the surface of undissolved salt and
in the rest of solvent volume. This is a kind of barrier with very complex physics if you would look on the equations. So, in the reality there is a
difference between actual concentration of solution which is in equilibrium with crystalls and concentration you try to measure (in the main volume of
the solvent).
That's why we can expect some difference from solubility results depending on the method of measurement.
[Edited on 8-3-2023 by teodor]
[Edited on 8-3-2023 by teodor]
|
|
|
yobbo II
National Hazard
   
Posts: 758
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Regarding the storm glass.
The solubility of stuff will depend on pressure, temperature and the pressure of water vapour (the humidity).
The stuff in the storm glass must be very sensitive to these changes.
I think.
|
|
|
Rainwater
National Hazard
   
Posts: 913
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
I want to provide an example i think is revelant to this discussion, which lead me to a lot of good studies reguarding solubility.
While back I made some silver chloride with unusual results.
A perfectly clear solution. It stumped me for a few days.
I finally asked my professor, she laughed and recommended I read
Quantitative Chemical Analysis
by: Harris, Daniel C.
ISBN: 9781429218153
If i really wanted to figure out what happened.
This book corrected my understanding of what really happens when a compound dissolves in another.
This book also contains step by step procedures and mathematics required for the experiments the OP suggest.
My AgCl Notes:
Reaction:
$$ KNO_3(aq) + HCl(aq) + Ag(s)\leftrightarrow K^+ + H^+ + NO_3^- + Cl^- + Ag$$
$$ 3 Ag(s) + 4 HNO_3(aq) \rightarrow 3 AgNO_3(aq) + 2 H_2O + NO(g)$$
$$ AgNO_3 + Cl^- \rightarrow AgCl(s) + NO_3^- $$
Lab notes
| Code: |
Test tube was weighed dry. 18.48 grams
2.31 grams of dry KNO3 added to test tube
About 1/3rd of di water was added to the test tube. (5mL)
Light heating was applied
A dry graduate cylinder was weighed 15.15 grams
2.61g of 32% hcl was weight in. 2.3ml was the volume
The test tube was heated until all the solids dissolved and a clear solution was obtained
T1500 2.40g of silver metal was added, no reaction observed
The hcl was added.
T1505 the metal begain to darken. The solution is gently boiling
T1535 an additional 2.30g of KNO[sub]3[/sub] and 2.3ml of HCl was added
T1555 the smell of no[sub]2[/sub] is coming from the end of the test tube
T1605 scraped this crap. Found my bottle of nitric acid
3 days later, the test tube contained a clear solution, which turned white when I picked it up.
|
[Edited on 9-3-2023 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
Texium
Administrator
       
Posts: 4566
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by teodor  | Did somebody observe the phenomenon when on long standing some saturated solutions of salts in water deposit crystalls and this is impossible to
explain by temperature just because the deposit doesn't disappear during further temperature fluctuations?
The standard method of measuring solubility includes agitation of crystalls and this is also some energy which also participates in the equilibrium
even if we keep temperature constant.
The mechanism of crystallization from saturated solution goes through some step of creating pre-crystal film with different (lower) solution
concentration. So, in normal equilibrium there are always 2 zones with different concentrations of solution - on the surface of undissolved salt and
in the rest of solvent volume. This is a kind of barrier with very complex physics if you would look on the equations. So, in the reality there is a
difference between actual concentration of solution which is in equilibrium with crystalls and concentration you try to measure (in the main volume of
the solvent).
That's why we can expect some difference from solubility results depending on the method of measurement. |
Excellent point! That’s something I’ve definitely observed but didn’t know exactly how to describe. IIRC, making a saturated
ammonium chloride solution gave me a really hard time because of that issue, though the resultant crystals were very beautiful.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 165
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|

I've observed that anhydrous copper sulphate rehydrates instantly on contact with water, which raises more questions about the semantics of what the
solubility of anhydrous copper sulphate actually means if it can't coexist with water.
Presumably it's based on the formula in my original post, although I can't find any sources with data that reflect it very well, except for the Crystal Growing wiki and Sigma Aldrich table, which come pretty close except for the 100°C data point and the fact that Ligma Aldrich's hydrate and anhydrate data appear
to be swapped.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4320
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by SnailsAttack  |
I've observed that anhydrous copper sulphate rehydrates instantly on contact with water, which raises more questions about the semantics of what the
solubility of anhydrous copper sulphate actually means if it can't coexist with water. |
It just means that it's the amount of copper(II) sulphate in a saturated solution. The degree of hydration it came in with just isn't relevant.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 165
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by DraconicAcid  | | It just means that it's the amount of copper(II) sulphate in a saturated solution. The degree of hydration it came in with just isn't relevant.
|
If you can find any data online where the solubility of the hydrate and anhydrate are correlated by an actual formulaic expression, please share.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 165
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
I came up with a method of measuring the solubility of copper sulphate (anhydrate or hydrate) using only mass measurements. The procedure is as
follows:
| Quote: |
Step 1. Soak a ~10 gram sample of copper sulphate (anhydrate or hydrate) in ~25 mL of water for ~10 minutes, stirring occasionally.
Approximately half the copper sulphate will dissolve at room temperature.
Step 2. Decant most of the copper sulphate solution and record the solution's temperature and mass (m ₜ ₜ ₗ).
Step 3. Evaporate the copper sulphate solution and weigh the resulting pentahydrate crystals (m ₕ). To ensure they're fully dry,
break them up occasionally, taking multiple measurements over time to ensure they stop losing mass.
|
This method relies on my observation that copper sulphate solution evaporates to form a stoichiometric pentahydrate, and will have to be modified to
work for more finicky salts.
I devised the following formula (based on the one in the original post, not backed up by empirical data) to compute the solubility of any hydrate
(s ₕ) from the mass of a saturated solution (m ₜ ₜ ₗ) and the mass of dissolved hydrate (m ₕ):
s ₕ = m ₕ·d ₛ /(m ₜ ₜ ₗ + m ₕ(Mᵣ - 2))
Where:
s ₕ is the solubility of the hydrated salt in g/L of solvent at a given temperature.
m ₕ is the mass of the solvated hydrated salt in grams.
d ₛ is the density of water at a given temperature.
m ₜ ₜ ₗ is the mass of the saturated solution at a given temperature.
Mᵣ is the anhydrate/hydrate molar mass ratio.
I performed this procedure and did the calculations for a sample of copper sulphate pentahydrate, and recorded the results below.
| Quote: | = Copper sulphate pentahydrate solubility test
Mass of solution (m ₜ ₜ ₗ): 18.579 ± 0.010 g
Temperature of solution: 19.0 ± 0.5°C
Mass of dissolved CuSO₄·5H₂O (m ₕ): 4.469 ± 0.005 g
The density of water at 19°C is 998 kg/L. The molar mass ratio of anhydrous to hydrous copper sulphate is 159.61/249.69 = 0.63923.
s ₕ = m ₕ·d ₛ /(m ₜ ₜ ₗ + m ₕ(Mᵣ - 2))
s ₕ = 4.469·998/(18.579 + 4.469(0.63923 - 2))
s ₕ = 4460/(18.579 + 4.469(-1.36077))
s ₕ = 4460/12.498
s ₕ = 356.9 ± 6.1 g/L of solvent
Predicted hydrous solubility based on data: 351 ± 7 g/L (extrapolated from anhydrate data)
Deviation from theoretical: 1.7% |
The solubility formula and measurement procedure appear to work, but it's kind of hard to say for sure since I don't have a reliable source to
reference against.
The total list of hydrate solubility equations that I've come up with are given below. At the moment they're not backed up with empirical data, except
for the formula correlating m ₕ to mₐ which is basic stoichiometry.
s ₕ = sₐ/(Mᵣ + sₐ(Mᵣ - 1)/1,000)
m ₕ = mₐ/Mᵣ
sₐ = mₐ·d ₛ /(m ₜ ₜ ₗ - mₐ/Mᵣ) = m ₕ·Mᵣ·d ₛ /(m ₜ ₜ ₗ - m ₕ)
s ₕ = m ₕ·d ₛ /(m ₜ ₜ ₗ + m ₕ(Mᵣ - 2)) =
mₐ·d ₛ /(m ₜ ₜ ₗ·Mᵣ + mₐ(Mᵣ - 2))
Where:
s ₕ is the solubility of the hydrated salt in g/L of solvent at a given temperature.
sₐ is the solubility of the anhydrous salt in g/L of solvent at a given temperature.
Mᵣ is the anhydrate/hydrate molar mass ratio.
--
m ₕ is the mass of the solvated hydrated salt in grams.
mₐ is the mass of the solvated anhydrous salt in grams.
--
d ₛ is the density of water at a given temperature.
m ₜ ₜ ₗ is the mass of the saturated solution at a given temperature.
|
|
|
Sulaiman
International Hazard
    
Posts: 3678
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by SnailsAttack  |
I've observed that anhydrous copper sulphate rehydrates instantly on contact with water, which raises more questions about the semantics of what the
solubility of anhydrous copper sulphate actually means if it can't coexist with water. |
so,,,
if anhydrous is added to a saturated solution,
it will precipitate as pentahydrate?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 165
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by yobbo II  | Regarding the storm glass.
The solubility of stuff will depend on pressure, temperature and the pressure of water vapour (the humidity).
The stuff in the storm glass must be very sensitive to these changes. |
Not possible. It's a sealed, rigid container, only the external temperature is capable of affecting the solubility.
Quote: Originally posted by Rainwater  | I want to provide an example i think is revelant to this discussion, which lead me to a lot of good studies reguarding solubility.
While back I made some silver chloride with unusual results.
A perfectly clear solution. It stumped me for a few days. |
In the past I've observed some salt mixtures that should result in a metathesis reaction but inexplicably fail to produce a precipitate unless the
solution is evaporated to an absurd concentration.
What do you know about how this works? I've been calling it 'quasi-stability'.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 165
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | Quote: Originally posted by SnailsAttack  | | I've observed that anhydrous copper sulphate rehydrates instantly on contact with water, which raises more questions about the semantics of what the
solubility of anhydrous copper sulphate actually means if it can't coexist with water. |
so,,,
if anhydrous is added to a saturated solution,
it will precipitate as pentahydrate? |
Yeah, I read your original question about this and I'm pretty sure the anhydrate would pull water from solution to form the pentahydrate, but fail to
dissolve due to insufficient water. I think some of the solvated copper sulphate would also crash out as pentahydrate since the anhydrate will have
absorbed a portion of the water that was keeping it in solution.
It's worth noting that the hydration of anhydrous copper sulphate is very exothermic (to the point that small quantities of water actually boil on
contact), so that would briefly raise the solubility.
|
|
|
Rainwater
National Hazard
   
Posts: 913
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
https://www.academia.edu/31178443/Harris_Quantitative_Chemic...
starting on page 121
of the book i references above, some useful google terms are
Solubility product = Ksp
Disproportionation
Common ion effect
Coprecipitation
Complex formation
Simple version.
When you have "Pure" H2O + CuSO4
You have every combination of cations and anions in solution.
H+, OH-, CuOH, H2SO4, etc etc etc. Lots of combination.
But each combination is interacting in its own little area of the solution until an
equilibrium is reached.
Applied to original post, from dry to wet
Starting with anhydrous CuSO4 and adding 1 molar of water, one would think you
would end up with the monohydrate. But without the ability to distribute the water
evenly on an atomic level, a variety of hydrates will be formed.
From wet to dry, as in measuring a percipitate, separating the hydrate from pure
water is a combination of which compounds percipitate first and how much.
Now lets leave the ideal behind and now consider all the N2, O2, CO2 and everything
else found in water. The combinations start adding up, and interactions get complex
Now the context of these example is from the prospective of reducing
measurement errors to as close to zero as possible, then using a hot mess of
formulas to know how much will remain in solution, so that measurements can be
corrected and total masses calculated.
And all that very improperly sums up the first 6 chapters, 142 pages
Edit:
| Quote: | | Maybe. Could be tough to maintain a 100°C bath anyways. |
Double boiler. Will get you close, a pressure vessel will get you perfect, but that sets the difficulty bar up to max by introducing another variable
no one has mentioned yet. Pressure.
Higher pressure will decrease the entropy, empathy damnit, .... disorder ... of the system, effecting the equilibrium.
Hope your good at spreadsheets
[Edited on 11-3-2023 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
yobbo II
National Hazard
   
Posts: 758
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Sounds simply enough!
There is a paper here
http://www.sciencemadness.org/talk/files.php?pid=682195&...
showing the system CuSO4 water from 0 to 100C (APPROX).
It may be of use.
The more I read the less I know.
Yob
|
|
|
Tsjerk
International Hazard
    
Posts: 3031
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by SnailsAttack  | Quote: Originally posted by DraconicAcid  | | It just means that it's the amount of copper(II) sulphate in a saturated solution. The degree of hydration it came in with just isn't relevant.
|
If you can find any data online where the solubility of the hydrate and anhydrate are correlated by an actual formulaic expression, please share.
|
You mean a formula that tells you how soluble the anhydrate is compared to the hydrate? You either divide or multiply by the ratio between the
molecular weights.
If 10 grams of the anhydrate dissolves in 100 ml solution you divide by the molecular weight of the anhydrate and multiply by the molecular weight of
the hydrate (multiply by 1.56). The other way around would be division by 1.56.
I think the question whether the starting hydration of a salt influences the final solubility can be answered by the fact there are no sources stating
different solubilities for the two. Just different solubilities from different sources. Doesn't that tell you solubility is just hard to measure?
Quote: Originally posted by SnailsAttack  | | Despite being an apparently simple topic, the solubility behavior of hydrated salts compared to their anhydrates (and associated anomalies) remain
undocumented and unexplained. |
The difference in behaviour is undocumented because there is no difference, the differences between sources is explained by the difficulty of
measurement.
When you think about a system that contains copper sulfate both dissolved and undissolved and is in equilibrium (saturated solution) all there is is
water, copper sulfate in solution and fully hydrated solid copper sulfate. How would the hydration state of the starting copper sulfate influence the
final concentration?
In chemistry, when something is not there, it can't influence the state of what is there. The hydration state of the starting material is not there
anymore, so it can't influence the equilibrium, can it?
[Edited on 11-3-2023 by Tsjerk]
|
|
|
Texium
Administrator
       
Posts: 4566
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Tsjerk  | | The difference in behaviour is undocumented because there is no difference, the differences between sources is explained by the difficulty of
measurement. |
At this point, I have given up on trying to argue reason in this thread, and I figure we may as
well just sit back and watch SnailsAttack attempt to reinvent the wheel. It could be entertaining.
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I think this is the correct table (and it is published in 1894):
https://gallica.bnf.fr/ark:/12148/bpt6k34902q/f552.item
There are 2 reasons why some sources contain incorrect data for CuSO4.
1. Solubility curve of CuSO4 has 3 anomalies in the pentahydrate range: 28.9, 34.85, 53.72C. It is like spikes where properties of crystalls are
changing very fast in very narrow range (fractions of degree) and comes back to the curve:
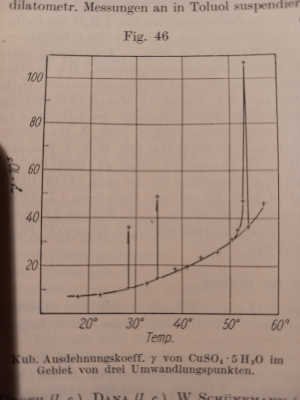
There is a reason for this behaviour: water molecules in crystalls change behaviour from oscilation to rotation. But those anomalies of CuSO4
solubility were discovered in the end of 19 centure. Try to evaluate the quality of your in-house methods. And probably what you can observe at those
points is "there is no constant equilibrium".
Solubility table in classical references are very often just interpolations, for example Bronsted (1928) gives the full table built by 4 actual
measurement, the rest is calculated by generic formula.
2. After 95.9C it doesn't crystallize as pentahydrate, so those table which gives solubility at 100C can incorrectly substract 5 mols of water instead
of 3.
So, for me there is no any miracle here.
[Edited on 12-3-2023 by teodor]
|
|
|
Sulaiman
International Hazard
    
Posts: 3678
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Maybe not a miracle but certainly a revelation.
Two obvious questions:
1 is this common behaviour for hydrated ions/molecules?
(eg MgSO4, Cu(NO3) 2 etc)
2 can I use this effect to create a hyper-saturated copper sulphate solution ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Texium
Administrator
       
Posts: 4566
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
1. Probably.
2. Doubtful, because it requires the temperature to be held at an exact value with a precision of 0.1 degrees.
[Edited on 3-12-2023 by Texium]
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 165
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | You mean a formula that tells you how soluble the anhydrate is compared to the hydrate? You either divide or multiply by the ratio between the
molecular weights.
If 10 grams of the anhydrate dissolves in 100 ml solution you divide by the molecular weight of the anhydrate and multiply by the molecular weight of
the hydrate (multiply by 1.56). The other way around would be division by 1.56. |
If you do the math, your formula doesn't make any sense. I've got it written as follows:
s ₕ = sₐ / Mᵣ
Where:
s ₕ is the solubility of the hydrated salt in g/L of solvent at a given temperature.
Mᵣ is the anhydrate/hydrate molar mass ratio.
sₐ is the solubility of the anhydrous salt in g/L of solvent at a given temperature.
Let's assume the solubility of the anhydrous salt at 20°C is 205 g/L. Therefore the solubility of the hydrate is: 205/0.63923 = 320.70 g/L.
| Quote: |
320.70 grams of CuSO₄·5H₂O dissolves in 1 liter of solvent (998 grams of water).
Total CuSO₄: 205.00 g
Total water: 115.70 + 998 g
Water/salt ratio: 5.433 or 184.1 g/L
205.00 grams of CuSO₄·0H₂O dissolves in 1 liter of solvent (998 grams of water).
Total CuSO₄: 205.00 g
Total water: 998 g
Water/salt ratio: 4.868 or 205.0 g/L
|
The solubility of the anhydrate comes out 10% higher, which would suggest that it's somehow intrinsically more soluble than the hydrate, which we both
agree isn't possible. The water/salt ratio is balanced in the formula I already discussed in the original post.
Quote: Originally posted by Texium  | | At this point, I have given up on trying to argue reason in this thread, and I figure we may as well just sit back and watch SnailsAttack attempt to
reinvent the wheel. It could be entertaining. |
Your guys' wheel is a friggin octagon at best!
|
|
|
| Pages:
1
2
3
4 |