| Pages:
1
2 |
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Problems detonation of the mixture PETN/NG
Hello friends, I recently decided to test the composition of PETN:NG
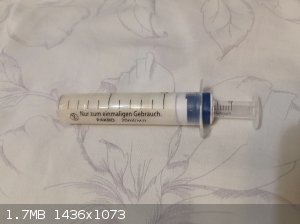
You can see the syringe in the photo.
Its upper, darker layer of 17.5 ml is a mixture of PETN:NG (80:20) 27g. Mixed at room temperature and compressed to a density of 1.55 g/cm3. Dry PETN
is pressed on top at a density of 1.25. A little over 5 grams.
A small, insulin syringe is a detonator cap, in the photo it is empty, but later 1g PETN was pressed into it at a density of 1.1 and 0.7g HMTD.
On the following pictures you can see the result. In my opinion, the damage is too weak. Thinking about it, only one option comes to me, the finished
detonator cap was wrapped with tape, because of which I could not fully insert it into the main charge. There was a gap of up to 1 cm.
But isn't 1g of PETN capable of initiating the detonation of dry PETN, very lightly pressed, at a distance of only 1 cm? As far as I know, PETN is not
at all prone to deflagration, and if it does explode, then completely, at the highest possible detonation velocity at an available density. And if
these 5g exploded completely, then the main 27g with nitroglycerin should have exploded completely. Or am I wrong?
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 403
Registered: 12-10-2022
Location: Due South of Due West
Member Is Online
Mood: Like a pendulum
|
|
How was the charge attached to the target? How pure is your PETN? Was your NG dried? Also, NG can can detonate at a low VoD; perhaps the mixture can
too. In this sort of thing there is many variables.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sir_Gawain  | | How was the charge attached to the target? How pure is your PETN? Was your NG dried? Also, NG can can detonate at a low VoD; perhaps the mixture can
too. In this sort of thing there is many variables. |
I believe that both substances were pure.
PETN was washed with two tens of liters of cold and hot water, after which it was boiled in a 1% solution of baking soda until the formation of foam
ceased (destruction of sulfoesters).
Then it was recrystallized from boiling acetone with the addition of baking soda (destruction of intracrystalline acid). It was recrystallized by
dropping water into an acetone solution to obtain large, well-compressible crystals.
The nitroglycerin was washed successively with 1.5 volumes of cold, warm water, followed by 1.5 volumes of 3% sodium bicarbonate solution, and again
with water. Rinse until indicator paper shows pH 6. Flushing was carried out with an aquarium compressor.
After that, nitroglycerin was settled for 2 days
The charge was attached to the fence section with cable ties.
[Edited on 30-7-2023 by DennyDevHE77]
|
|
|
Microtek
National Hazard
   
Posts: 861
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Ester formation is a reversible equilibrium reaction, so boiling it with water and anything that can catalyse the reaction will substitute nitrate
groups with hydroxyl groups. If too much acid is present in the crude PETN, this may have happened.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | | Ester formation is a reversible equilibrium reaction, so boiling it with water and anything that can catalyse the reaction will substitute nitrate
groups with hydroxyl groups. If too much acid is present in the crude PETN, this may have happened. |
Practice shows that PETN is hydrolyzed very weakly, it must be boiled for a really long time, or in an acidic solution.
Molecules of pentaerythritol with hydroxyl groups are soluble in water, and if something like this happened, they would go into the water.
So I have no doubts about the purity of my PETN. Pentaerythritol sulfates are destroyed by boiling in water, undernitrated PE disappears after
repeated washings, and intracrystalline acid is neutralized in acetone.
The only thing that could reduce the power is the presence of up to 5% admixture of dipentaerythritol in technical pentaerythritol, which, upon
nitration, will turn into hexanitrodipentaerythritol. Which is a little weaker than PETN.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1382
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Damage looks like full detonation only 5g. With 32g, the beam should be broken into two pieces. But there are a lot of variables, as said. Any
distance in millimeters from the steel reduces the effect.
....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
PLSHY
Hazard to Self
 
Posts: 91
Registered: 30-7-2023
Member Is Offline
|
|
Your nitration process is excellent, I believe it is very pure PETN and NG, but NG has a very important problem, which is low velocity detonation. As
far as I know, even 10 grams of loose petn is not enough to trigger the high-speed detonation of EGDN, yes, it is EGDN! This is the result of my own
experiments. If you want to trigger the high-speed detonation of EGDN, you need to detonate the high-detonation velocity and high-pressure charge
such as molten cast ETN, but if NG is mixed with diatomaceous earth, it will not cause low-speed detonation, but I don’t know if it can be mixed
with PETN. Low velocity detonation. Or the arrival of petn will make the viscosity of NG higher (we all know that the viscosity of liquid explosives
is a key indicator for determining high-speed detonation), making it more difficult for high-speed detonation. I have not carried out relevant
experiments, nor do I know the relevant knowledge, but I can provide such an idea (that is, a low-velocity detonation may have occurred) for reference
only.
|
|
|
PLSHY
Hazard to Self
 
Posts: 91
Registered: 30-7-2023
Member Is Offline
|
|
Your nitration process is excellent, I believe it is very pure PETN and NG, but NG has a very important problem, which is low velocity detonation. As
far as I know, even 10 grams of loose petn is not enough to trigger the high-speed detonation of EGDN, yes, it is EGDN! This is the result of my own
experiments. If you want to trigger the high-speed detonation of EGDN, you need to detonate the high-detonation velocity and high-pressure charge
such as molten cast ETN, but if NG is mixed with diatomaceous earth, it will not cause low-speed detonation, but I don’t know if it can be mixed
with PETN. Low velocity detonation. Or the arrival of petn will make the viscosity of NG higher (we all know that the viscosity of liquid explosives
is a key indicator for determining high-speed detonation), making it more difficult for high-speed detonation. I have not carried out relevant
experiments, nor do I know the relevant knowledge, but I can provide such an idea (that is, a low-velocity detonation may have occurred) for reference
only.
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 280
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
I know NC and NG when mixed can have a confusingly low VoD and high critical diameter. Maybe this is the case for PETN/NG as well?
Regardless, your purification via boiling in baking soda solution is unusual to me. Simple dissolve and crash recrystallization does a good job of
removing any acids, sulfates and lower nitrates.
Dissolve dry crude PETN into hot acetone until near saturation, pour into bicarb solution, filter it out and repeat once or twice more with pure
water. It's simple and works very well.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PLSHY  | | Your nitration process is excellent, I believe it is very pure PETN and NG, but NG has a very important problem, which is low velocity detonation. As
far as I know, even 10 grams of loose petn is not enough to trigger the high-speed detonation of EGDN, yes, it is EGDN! This is the result of my own
experiments. If you want to trigger the high-speed detonation of EGDN, you need to detonate the high-detonation velocity and high-pressure charge
such as molten cast ETN, but if NG is mixed with diatomaceous earth, it will not cause low-speed detonation, but I don’t know if it can be mixed
with PETN. Low velocity detonation. Or the arrival of petn will make the viscosity of NG higher (we all know that the viscosity of liquid explosives
is a key indicator for determining high-speed detonation), making it more difficult for high-speed detonation. I have not carried out relevant
experiments, nor do I know the relevant knowledge, but I can provide such an idea (that is, a low-velocity detonation may have occurred) for reference
only. |
Thank you, many consider this nitration process redundant! And I didn’t know that a mixture of PETN with liquid nitroesters is so hard to start, in
truth, all I know about this mixture is a few pages from Urbański, where such mixtures were mentioned called penthrinites (or hexonites if RDX was
taken instead of PETN), their speed was indicated, easier compressibility, and desirable ratios of PETN/Nitroglycerin, sometimes nitrocellulose, and
that they were developed by Stetbacher. In Stetbacher's book "gunpowder and explosives" there is not much information about them either.
In particular, this 80/20 composition in a 20 cc syringe, I was able to press in above 1.5 by hand. While coarse-grained PETN was dry pressed to 1.3.
And fine PETN obtained by pouring acetone into water was pressed to 0.9.
I also thought that like the classic kieselguhr dynamite, NG impregnated with powder, especially PETN, will cock just as easily or even easier.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by OneEyedPyro  | I know NC and NG when mixed can have a confusingly low VoD and high critical diameter. Maybe this is the case for PETN/NG as well?
Regardless, your purification via boiling in baking soda solution is unusual to me. Simple dissolve and crash recrystallization does a good job of
removing any acids, sulfates and lower nitrates.
Dissolve dry crude PETN into hot acetone until near saturation, pour into bicarb solution, filter it out and repeat once or twice more with pure
water. It's simple and works very well. |
Yes, Stetbacher also pointed out, like Naum, that gelignite degrades in a week or two and gives out about 1700-2000 m / s. True, the same Shtetbacher
indicates that adding only 10% of a powerful blasting explosive like RDX or PETN to the explosive jelly makes the jelly always detonate at maximum
speed
And PETN was not invented by me to boil in an alkaline solution. Books that describe the obsolete two-stage process for obtaining PETN indicate that
PETN was boiled in autoclaves to remove sulfates. I do this with baking soda just for convenience. Sodium carbonate can also be used, but it is too
alkaline and the temperature must not be raised above 90°C, otherwise PETN will begin to decompose. Or you can take magnesium oxide, but then you
need to separate it. Sometimes I use 99% nitric acid with a small amount of carbamide added. After her PETN, I just boil for a few minutes in acetone
with soda.
|
|
|
Tsjerk
International Hazard
    
Posts: 3031
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Bicarbonate decomposes readily to carbonate upon boiling. You will lose CO2, so you will end up with half of it, but still. 1 molar bicarbonate has a
pH of around 8-9, a half molar solution of carbonate has a pH of around 10.
[Edited on 1-8-2023 by Tsjerk]
|
|
|
Etanol
Hazard to Others
  
Posts: 179
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Soda does not seem to work in acetone. Ammonium bicarbonate or concentrated ammonia works.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Could you give an argument why soda should not work? Sodium carbonate and bicarbonate are widely used in industry, moreover, they are even more
preferred, since ammonium carbonate gives many by-products.
For example, ammonium carbonate causes partial condensation of acetone to form mesityl oxide, which in turn reacts with ammonia, which is obtained
from ammonium carbonate, forming diacetonamine. It is also obtained simply from acetone and ammonia through the condensation of acetonamine.
Also, ammonia and carbon dioxide condense back into ammonium carbonate, which can clog communications.
So in the industry, soda looks preferable.
And at home, of course, you can use both.
|
|
|
Etanol
Hazard to Others
  
Posts: 179
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DennyDevHE77  |
Could you give an argument why soda should not work? Sodium carbonate and bicarbonate are widely used in industry, moreover, they are even more
preferred, since ammonium carbonate gives many by-products.
For example, ammonium carbonate causes partial condensation of acetone to form mesityl oxide, which in turn reacts with ammonia, which is obtained
from ammonium carbonate, forming diacetonamine. It is also obtained simply from acetone and ammonia through the condensation of acetonamine.
Also, ammonia and carbon dioxide condense back into ammonium carbonate, which can clog communications.
So in the industry, soda looks preferable.
And at home, of course, you can use both. |
Sodium bicarbonate is well soluble in water, but not soluble in acetone. I tested the acidity of PETN acetone solutions: sodium bicarbonate is not
able to destroy all acids and sulphoesters for 30 minutes. Ammonia makes a solution neutral in a few seconds.
In industry, sodium carbonate and bicarbonate are used in aqueous solution, ammonia and ammonia carbonate are used in water and acetone solution.
Acetonomin is unstable in the presence of water if I remember correctly. It's not a problem.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
PETN needs to be heated in acetone, and ideally boiled (it's faster this way). Destruction of sulfonic esters will be manifested by a change in
solution to dark yellow. Sodium bicarbonate in acetone is very slightly soluble, tenths of a gram, but this is apparently enough, otherwise it would
not be used, especially since water is added dropwise to acetone, so that even more of the soda will dissolve.
I will give a recipe taken from the book "Chemistry and Technology of High Explosives" by Orlova E.U.:
"Acetone in the amount of 360 liters from the storage is fed with compressed air (or nitrogen) into an automatic measuring tank, and from it, through
a safety pot, into the heater." where it is heated up to 50°C. Then acetone enters the solvent, where 112 kg of wet (or 100 kg of dry) PETN and 750 g
of sodium bicarbonate are loaded. After dissolution of PETN (30 min), the solution is drained through the filter into the crystallizer, where another
750 g of sodium or ammonium bicarbonate is added. Sodium bicarbonate is introduced in two stages in order not to create a highly alkaline environment
and to avoid corrosion of aluminum equipment. To the acetone solution in the crystallizer, 600 liters of cold water are gradually added from a
measuring cup at a rate of 30 l / min "with the mixer running. With this mode of precipitation, PETN with good flowability is obtained"
[Edited on 7-8-2023 by DennyDevHE77]
|
|
|
Microtek
National Hazard
   
Posts: 861
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I don't know this publication and so, can't really comment on the quality. There are some red flags in this excerpt, however:
"Then acetone enters the solvent...". This doesn't really make sense since there is no mention of which solvent is referred to, and from the context
it would seem that only PETN and possibly water is present beside the acetone.
More importantly, I see no mention of sulfoester destruction here. This is just a variation of the technique that everybody here has been practicing
for ages: Dissolve the crude PETN in acetone, contact the solution with a mild base such as bicarbonate to ensure complete neutralization of acid
residue trapped in the crystals and reprecipitate the PETN by dilution with water.
There are many variations you can do to achieve whatever crystal morphology you prefer, such as a free flowing one in this case.
|
|
|
Etanol
Hazard to Others
  
Posts: 179
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Have you checked pH? There should be stable pH8.
Color can be a sign of not only neutralization.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | I don't know this publication and so, can't really comment on the quality. There are some red flags in this excerpt, however:
"Then acetone enters the solvent...". This doesn't really make sense since there is no mention of which solvent is referred to, and from the context
it would seem that only PETN and possibly water is present beside the acetone.
More importantly, I see no mention of sulfoester destruction here. This is just a variation of the technique that everybody here has been practicing
for ages: Dissolve the crude PETN in acetone, contact the solution with a mild base such as bicarbonate to ensure complete neutralization of acid
residue trapped in the crystals and reprecipitate the PETN by dilution with water.
There are many variations you can do to achieve whatever crystal morphology you prefer, such as a free flowing one in this case.
|
I'm sorry, the solvent meant an industrial unit in which dissolution occurs and not a liquid for dissolution.
Sulfoesters are not mentioned here, as this is a fragment about recrystallization, in this book the desulfurization process is described as suspension
of 1 part PETH in 9-10 parts of near-boiling (90°C) 1% sodium carbonate solution. Boiling in water removes most of the sulfoesters. And later, when
boiling in acetone, only their last residues are removed. Although both sodium bicarbonate and magnesium oxide are suitable for this. but the latter
is less convenient. But they can be boiled. This is how I usually clean PETN. And then into acetone.
Personally, I measured the pH with indicator paper, although I did this already when the finished PETN was re-dissolved. pH was about 8. But it seemed
to me that this was a lot (I made pentolite) and to protect against potential troubles, I then acidified it with a small amount of citric acid
[Edited on 7-8-2023 by DennyDevHE77]
|
|
|
Etanol
Hazard to Others
  
Posts: 179
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DennyDevHE77  |
Personally, I measured the pH with indicator paper, although I did this already when the finished PETN was re-dissolved. pH was about 8.
|
Curiously
This sequence is acceptable.
Although this description of the purification refers to a one-stage synthesis from 90-99% nitric acid. Such a product does not contain mixed
nitro-sulfo esters initially.
They write about the two-stage method that the PETN requires "special stabilization".
[Edited on 7-8-2023 by Etanol]
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Etanol  | Quote: Originally posted by DennyDevHE77  |
Personally, I measured the pH with indicator paper, although I did this already when the finished PETN was re-dissolved. pH was about 8.
|
Curiously
This sequence is acceptable.
Although this description of the purification refers to a one-stage synthesis from 90-99% nitric acid. Such a product does not contain mixed
nitro-sulfo esters initially.
They write about the two-stage method that the PETN requires "special stabilization".
[Edited on 7-8-2023 by Etanol] |
Sorry, I didn't understand what you mean. This purification method (treatment with a soda solution + treatment with acetone) refers to the
purification of PETN obtained by a two-stage method, or one-stage using a sulfuric-nitric acid mixture.
Obviously, PETN synthesized using WFNA does not need to remove sulfoesters due to their absence. The usual processing in acetone is sufficient here.
Moreover, some grades of PETN are simply ground with calcium carbonate, bypassing the purification step in acetone, and get a not so perfect, but
still a good product.
[Edited on 7-8-2023 by DennyDevHE77]
|
|
|
Etanol
Hazard to Others
  
Posts: 179
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DennyDevHE77  |
This purification method (treatment with a soda solution + treatment with acetone) refers to the purification of PETN obtained by a two-stage method,
or one-stage using a sulfuric-nitric acid mixture.
|
to one-stage using a nitric acid only (WFNA).
Without sulfur acid!
[Edited on 7-8-2023 by Etanol]
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Etanol  | Quote: Originally posted by DennyDevHE77  |
This purification method (treatment with a soda solution + treatment with acetone) refers to the purification of PETN obtained by a two-stage method,
or one-stage using a sulfuric-nitric acid mixture.
|
to one-stage using a nitric acid only.
Without sulfur acid!
[Edited on 7-8-2023 by Etanol] |
Then enough high-quality flushing and ordinary acetone. There, the main thing is to remove undernitrated pentaerythritol, and intracrystalline acid
|
|
|
Etanol
Hazard to Others
  
Posts: 179
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DennyDevHE77  |
Then enough high-quality flushing and ordinary acetone. There, the main thing is to remove undernitrated pentaerythritol, and intracrystalline acid
|
and to decompose trinitrate, may be. It is insoluble in water too.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
There are no problems with it, trinitrate (petrin) is liquid, and is easily removed during washings. There is even a method for obtaining petrin from
a dilute sulfur-nitrogen mixture at a low temperature. There he goes with PETN.
PETN mixed with PETRIN will be oily
[Edited on 7-8-2023 by DennyDevHE77]
|
|
|
| Pages:
1
2 |