| Pages:
1
2 |
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
Really, read it again carefully and check the yields with primary alcohols -0%.
Is it expensive? Yes.
Is it practical beyong a bench scale? Probably not.
But it IS selective viz. this glycol.
I just need to go find a similarly selective reagent that is not so exotic and dear.
|
It is still not practical compared to published alternatives: cyclodehydration of the piperidinyl glycol or reduction of 3-quinuclidinone. You are
going to a lot of effort to try to reinvent the inclined plane here.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
No, I am going to a lot of effort to avoid breaking the law.
If I knew how to get from the ketone to the chloride without passing through the alcohol I'd certainly use that route.
The cyclodehydration still looks woefully low yield, even with a better reagent than KmnO4 and a better dehydration catalyst it's not going to much
exceed 10% overall.
However 4-vinylpyridine is cheap and I have plenty of it already for making PVPCC.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
According to ACS a paper was presented recently at their Midwest meeting describing a regioselective chlorination of secondary alcohols using
AlCl3.6H2O in N-methylpyrrolidone with microwave assistance.
http://acs.confex.com/acs/mwrm06/techprogram/P35541.HTM
I am trying to obtain the full text.
Aluminum chloride hexahydrate isn't expensive, Screw the MW-assist rubbish and ditto the green solvent hogwash.
The example illustrated in the abstract was a benzylic hydroxyl, but the title does say secondary alcohols.
[Edited on 20-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
I think you're beating that proverbial dead horse with that approach.
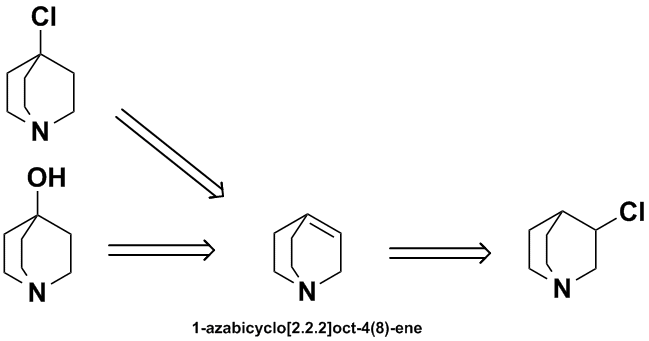
I don't have access to any quinuclidine chemistry review articles at the moment but both 4-chloro- & 4-hydroxyquinuclidine are known compounds.
Just blue-skying it from either of those starting points, you could imagine either a dehydration or a dehydrochlorination to give the
1-azabicyclo[2.2.2]oct-4(8)-ene, then add HCl under anti-Markovnikov conditions to get your 3-chloro compound. (I could find no reference to the
1-azabicyclo[2.2.2]oct-4(8)-ene so this method is either a) not technically possible or b) has not been described). The N-oxide derivatives may be
needed here. Access to the starting materials is another question.
[Edited on 20-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks. The effort to find a regioselective Chlorinating agent for secondary alcohols hasn't been entirely a waste of time. That AlCl3.6H2O is
promising, but not very well documented so far.
And the TMCE chemistry, though nonselective, is interesting and applicable to preparation of acyl chlorides, including formyl chloride, as well as
alkyl chlorides, bromides, iodides and even fluorides. So I will move the posts about it to another thread.
Furthermore the lit. also popped out a modification of the triphenylphosphine-CCl4 chlorination, in which hexachloroacetone is substited for CCl4. I
can't obtain CCl4 where I am for stupid reasons, but hexachloroacetone is no problem and not too expensive ($100 a liter Acros.) Again, this system
applies to both prep of alkyl halides and acyl halides and also anhydrides. So that's two reagent systems that have not been discussed on the forum
before.
I found a selective halogenating reagent for primary alcohols, as well. No help for the problem of this thread but maybe it will come in handy
sometime for some other synthetic dilemna. I will describe it as well, in the appropriate thread.
It is very clear from the literature that the search is on for regioselective halogenating reagents by many investigators. So, I will look into the
scheme you suggest above but I'll also continue the bastinado of the equine corpse as well, if you don't mind. I haven't even looked in several of the
best books I have for this sort of thing, like Katrizky's series on organic functional group transformations, Paquette's volume on organic reagents,
and so on.
Thanks again.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
So, I will look into the scheme you suggest above . |
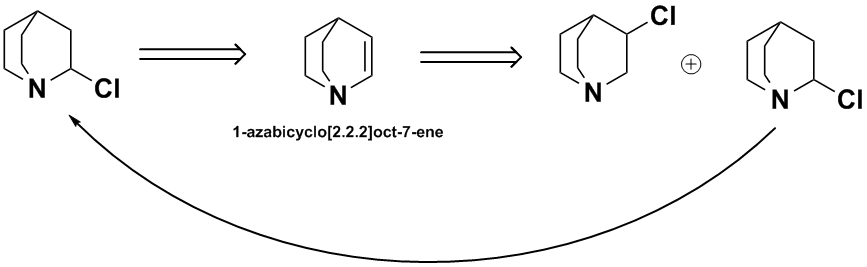
Here's a variation on my earlier suggestion. I found one reference to 2-chloroquinuclidine, so it is a known compound. But I found no reference to
1-azabicyclo[2.2.2]oct-7-ene though FMC prepared some 3-methanol derivatives of it.
The idea is to add HCl to the alkene, then separate the isomers & recycle the 2-chloro.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Again, thanks for the input. There ought to be lit. on the corresponding piperidine and chlorination of same. I'd expect the 2-chloro to predominate,
due to effect of the lone pair on the N.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
Again, thanks for the input. There ought to be lit. on the corresponding piperidine and chlorination of same. I'd expect the 2-chloro to predominate,
due to effect of the lone pair on the N. |
Perhaps the salt would shift the balance of the mixture. You would be adding HCl to an amine.
[Edited on 20-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
Again, thanks for the input. There ought to be lit. on the corresponding piperidine and chlorination of same. I'd expect the 2-chloro to predominate,
due to effect of the lone pair on the N. |
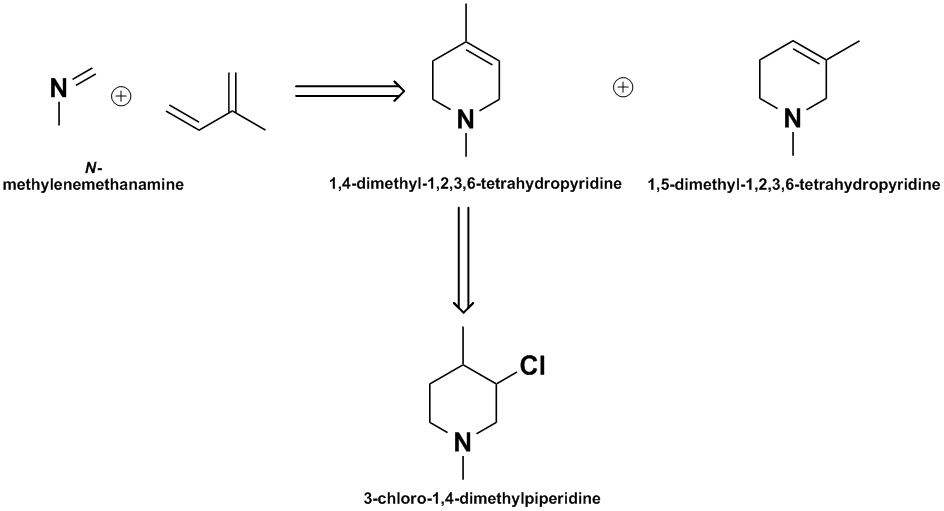
Here is some thoughts on the model piperidine system. The most direct approach to the terahydropyridine system seems to be via an aza-Diels-Alder
between isoprene & N-methylformidine, but then you have 2 positional isomers to contend with. I also have no references on the aldimine synthesis.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
| Pages:
1
2 |