LSD25
Hazard to Others
  
Posts: 239
Registered: 29-11-2007
Member Is Offline
Mood: Psychotic (Who said that? I know you're there...)
|
|
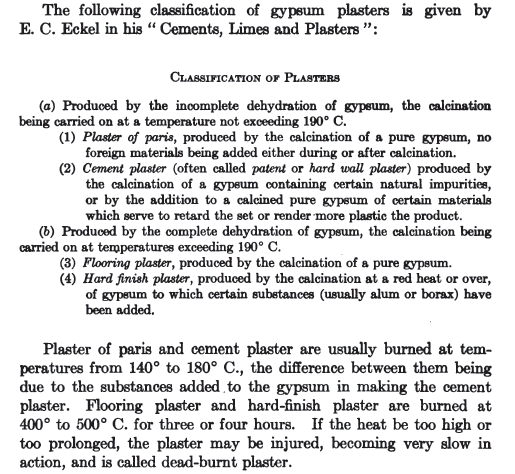
Dehydrating gypsum for cement-type use
I would like to know if anyone has any details on a useful, mid-scale preparative process for dehydrated gypsum?
I want it to use for a poured earth type recipe - 15-20% dehydrated gypsum, 80-85% earth (high aluminosillicate clay content) type as an alternative
building material.
Additionally, has anyone used this approach to building (poured or cast earth walls - using dehydrated gypsum instead of concrete)?
For the dehydration, I was thinking a wood or gas fired furnace/kiln setup - should I also be dehydrating the aluminosilicate?
PS I know this looks kinda out of place, but it don't really fit anywhere all that well, despite the fact that it is essentially a chemical process,
go figure - feel free to suggest moving the thread...
Whhhoooppps, that sure didn't work
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Well to fully dehydrate gypsum you only need to get it to 170c (per wikipedia). So really you could probably just bake a bunch in your oven for a
while maybe while leaving the door a crack open to allow water vapor to escape.
I am not positive what your intended usage is, but if it is to make a material that must be refractory, its not a good choice. Calcium is a powerful
flux at high temps, additionally at high temps calcium sulfate will decompose to calcium oxide. The only reason I say this is it looks like some poor
refractory mixes proposed on metal casting forums I lurk at.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
If you limit the heating to 120 C or a bit higher, you get Plaster of Paris. Taking it to 170 C gives you 'dead burnt' gypsum, which is very slow to
react with water.
Traditional poured earth mixes use Portland cement and soil. The more recent ones based on gypsum use lime and low fired gypsum, mixing line with soil
and water first, then adding the PoP as even with the lime additive it sets fairly quickly.
For use with gypsum is is suggested that the soil be no more than 15% clay, some say 10%; adobe uses about twice that much clay and Portland cement
poured earth requires a lower amount of clay similar to PoP. The Portland cement stabilised variety can tolerate more clay when being used for
flooring or walkways where it will not encounter heavy loads. Too much clay results in shrinkage while drying, causing extensive cracking.
Gypsum set poured earth is best for dry climates, as it leeches out of the mixture leaving just soil; for moist climates it must be waterproofed with
silicones, tar, waxes, or molten sulfur sprayed onto it.
http://www3.itu.edu.tr/~isikb/yemenbildiri01.html
http://www.formblock.com.au/f_a_q_.htm
http://www.sustainable.com.au/construction.html
http://www.waitakere.govt.nz/Abtcit/ec/bldsus/pdf/materials/...
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
| Quote: | Originally posted by not_important
If you limit the heating to 120 C or a bit higher, you get Plaster of Paris. Taking it to 170 C gives you 'dead burnt' gypsum, which is very slow to
react with water. |
I don't think that's true: I've reused investment several times. I use a mixture of 1 part (by volume) plaster of paris to 2 parts fine sand for
investment casting. The mold is fired to dull red heat, ensuring no residues of wax or chemically combined water. After the metal is poured and
solidified, the mold is quenched in water while still hot. The investment spalls off, loosening itself from the casting. After everything has
settled down, the water can be poured off and the plaster crud dried, baked and ground, bringing it back to the "plaster of paris" condition, ready
for reuse.
Gypsum dehydrates in two distinct phases, first around 150-200C to the hemihydrate, then around 600C to anhydrite. (Speaking of which, apparently
this occurs at moderate temperatures, just really slowly- anhydrite is a naturally occuring mineral.) Since calcium sulfate decomposes above 1100C, I
don't think it ever really goes "dead burnt". Not in my experience at least.
Tim
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Dead burnt is an old industrial term. Anhydrous CaSO4 very slowly reacts with moisture to rehydrate, but this can take weeks, if the temperature was
too high there is some sintering which slows the rehydration down even further.
For lump or massive gypsum the dehydration goes fairly slowly, the water escaping from the core keeping all bu the outermost layers slightly hydrated.
It's much quicker with powdered material, I accidentally nearly dead burnt some used PoP I was recycling by using 250C to dehydrate rather than 150,
the powder took 5 or 6 hours to set.
http://www.google.com/search?q=%22dead+burnt%22+plaster+OR+g...
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
for chromatography plates the form I'm using is "plaster of Paris" right? So if I used sheetrock, I'd dehydrate it at 120C?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemrox
for chromatography plates the form I'm using is "plaster of Paris" right? So if I used sheetrock, I'd dehydrate it at 120C? |
If you means as a binder, it's plaster of Paris. But I wouldn't use wallboard as a source, it's very likely to have bits of fiber, adhesive traces,
borates, wetting agents, and possibly other sulfates and organics in it. Buy a large sack of plain plaster of Paris, avoid some of the more
specialised plasters as they have other things besides CaSO4 in them.
|
|
|
LSD25
Hazard to Others
  
Posts: 239
Registered: 29-11-2007
Member Is Offline
Mood: Psychotic (Who said that? I know you're there...)
|
|
Back to the topic, I finally got time to have a look and I am considering going via the CaO/MgO route with monoammonium phosphate to get calcium
phosphate(s) and magnesium ammonium phosphate... This is actually one of the latest routes to phosphate cements, it is extremely strong within 1 hour.
Whhhoooppps, that sure didn't work
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I've only read of calcium phosphate cements in the context of biocements, where they are absorbed by the body, and magnesium based ones for
specialized applications such as rapid set and acid resistance. These are special applications with price premiums that are acceptable because of the
need for unusual properties.
The phosphate cements I've read of are not very tolerant of admixtures such as the aggregate typically used in concretes. The highest amounts I
recall seeing were 1/4 to 1/2 of the total mixture, and this was using fly-ash which is somewhat reactive in these systems, joining in with the other
cement reactions. Ordinary Portland cement when used in rammed or poured earth applications makes up 1/10 to 1/5 of the total with the soil being the
remaining 80 to 90 percent. Having to have the end product be 1/2 to 3/4 cement would seem to be more expensive than having 1/10 to 1/5 be cement.
The calcium based phosphate cements are loved by plants, who can break them down for the calcium and phosphate content. The high magnesium ones
inhibit plant growth, likely from the OD of Mg.
|
|
|