amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
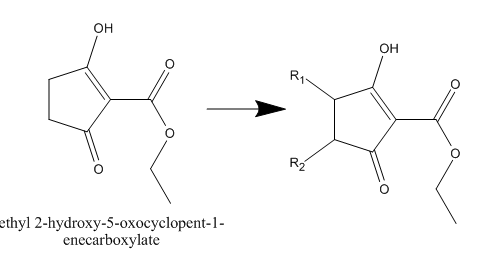
Synthesis of 4,5-disubstituted 1,3-cyclopentanediones
plz help me in this synthesis
I want to find way to make derivatives for this compound
[Edited on 7-3-2008 by amrhamed2]
<sub>Edit by Nicodem: Made the thread tittle fit the topic.</sub>
[Edited on 8/3/2008 by Nicodem]
amr h mahmoud
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
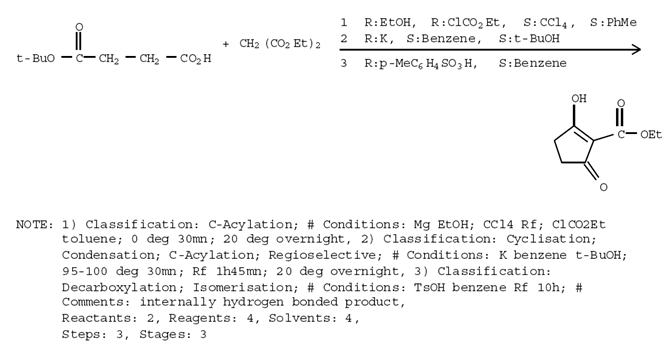
synthesis
here is the synthesis
[Edited on 7-3-2008 by amrhamed2]
amr h mahmoud
|
|
|
DNA
Hazard to Others
  
Posts: 191
Registered: 11-6-2003
Location: @moon
Member Is Offline
Mood: Experimenting
|
|
You could use the substance on the left the "ethyl 2-hydroxy-5-oxocyclopent-1-enecarboxylate" and brominate it with oxalyl dibromide.
Then you will have 2-Carbethoxy-3-bromocyclopentanon and with that you can go further since you have a halogen there.
Your question is by the way not completely clear could you elaborate.
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
sorry ...I really didn't make it clear ....I want to get the substituted derivatives with R1 and R2
U said bromination but I don't know how to make it selective for one site .....u see this compound can make Tautomerism and be symmetric diketone
amr h mahmoud
|
|
|
DNA
Hazard to Others
  
Posts: 191
Registered: 11-6-2003
Location: @moon
Member Is Offline
Mood: Experimenting
|
|
If you use oxalyl dibromide then the bromine will attach to where you have the OH written.
Here it is:
Journal; Buechi,G.; Weinreb,S.M.; JACSAT; J. Am. Chem. Soc.; EN; 93; 1971; 746-752.
As I am looking into the paper I just noticed that it replaces the OH and not coming on a R position
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I suggest looking upon some double deprotonated acetoacetate esters chemistry. There are a bunch of papers on this chemistry. Essentially, you doubly
deprotonate 1,3-dicarbonyl compounds making the less acidic position (position 4) the most nucleophilic, thus readily selectively alkylated. You can
then quench with ethyl chloroformate to add the EtOCO- group on the position 2 of 1,3-cyclopentandione. Alternatively, I would suggest preparing the
target compounds starting from appropriate acyclic compounds, that is preparing the appropriate 2,3-disubstituted succinanhydrides and continuing as
per that JOC reference.
[Edited on 7/3/2008 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
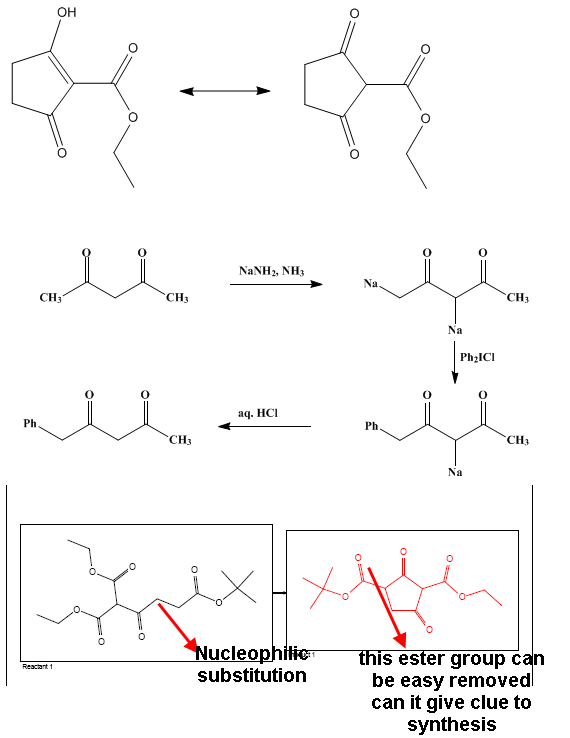
well sorry for my little information
I thought about deprotonation but the problem is that will substitution favor cyclization ....
(in the cyclic structure I think the probability is the same for R1 and R2 sites)
In order to carry out deprotonation ,it should be carried out before cyclization
Well I didn't get the quenching point
[Edited on 7-3-2008 by amrhamed2]
amr h mahmoud
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Actually, what I thought was to rather start with the commercially available 1,3-cyclopentadione (expensive, I know). Doubly deprotonate it (I think
it can be done in THF with 1 eq. NaH followed by 1 eq. BuLi; check the literature), alkylate it with whatever RX you have to put there and then quench
with EtOCOCl. Though, this would get you only one substituent on place.
I don't think you will get much reasonable replies unless you provide enough information for a reasonable answer. I can understand you are having some
troubles with the English language, but it doesn't look like you tried very hard. At the moment we don't even know what R1 and R2 are supposed to be.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
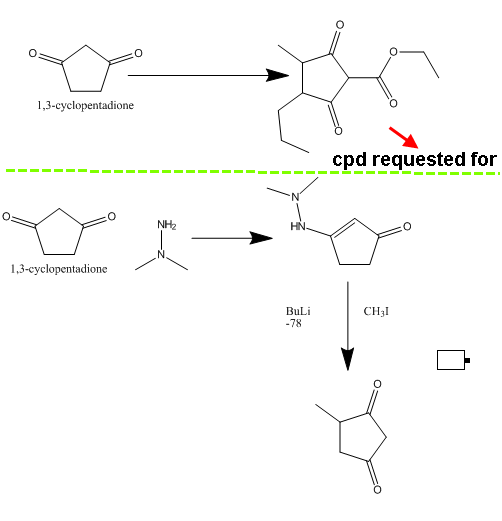
I will give an example for a compound requested ....let's say I want to prepare ethyl 3-methyl-2,5-dioxo-4-propylcyclopentanecarboxylate
To Nicoderm..I am not an expert but I searched hard and found many methods that can do monosubstitution (look below
Tetrahedron Lett.; EN; 30; 13; 1989; 1705-1708.) .
What I want is disubstitution .
The problem is that the ester group can be easily removed .
Can anyone help me?
[Edited on 7-3-2008 by amrhamed2]
[Edited on 7-3-2008 by amrhamed2]
[Edited on 7-3-2008 by amrhamed2]
amr h mahmoud
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
These might be useful papers to look up: Kobayashi, Y.; Taguchi, T.; Morikawa, T. Tetrahedron Lett. 1978,
3555. Kobayasi, Y.; Taguchi, T.; Morikawa, T.; Tokuno, E.; Sekiguchi,
S. Chem. Pharm. Bull. 1980,28, 262. and you could make the derivatives on the dicarboxylate enolate compounds and then do the oxidative
intramolecular coupling to the derivative cyclic diketone.. an approach anyway
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|