| Pages:
1
2
3
4 |
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Nice synthesis. I will try that out ASAP.
Check out the attachments woelen! The first one is from 1993 and has a detailed description on all chromium peroxo species.
The second one covers synthesis & structure of ammonium peroxychromate.
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Damn its 7Mb and it wont upload.
Here's the second one, at least. I can send you the other one via mail if you like.
Attachment: ammonium peroxychromate.pdf (265kB)
This file has been downloaded 1014 times
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Note that on the second page it states that the ammonium peroxochromate can only be kept for more than a year at freezing temperatures. Simply leaving
the crystals at room temperature will cause rapid decomposition (into the triamminediperoxochromium (IV) complex?) Although it is an interesting
synthesis, the necessity of working and storing the product at low temperatures makes it an impractical one.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
There are papers which say that the potassium salt decomposes at room temperature as well - but it obviously doesn't! woelen has kept his sample for
many years already.
There is a dissertation "Preparation, Structure and Vibrational Spectroscopy of Tetraperoxo Complexes of CrV+, VV+, NbV+ and TaV+" which gives a
detailed synthesis procedure for the ammonium salt. They stored it at -4°C yes. But they did the same with the potassium salt to "prevent possible
oxidation to chromate!". Altought we know that this is unnecessary because the salt IS stable at room temperature (given its completely dry, that is).
It also says that "All these compounds are very unstable as they decompose very rapidly at room temperature" - but this is not true, at least not for
the potassium salt. In fact the only decomposition at room temperature the author actually observed was that of ammonium tetraperoxovanadate,
(NH4)3[V(O2)4]. It seems like he was too lazy to investigate decomposition for all other tetraperoxo-compounds and stated that they would be similarly
unstable.
He has also recorded x-ray diffraction patterns which show the ammonium and potassium salt to be of the same structure, so it is very unlikely he has
mistaken the triammine complex for the ammonium salt. Thus I'm quite sure the ammonium salt DOES exist and it CAN be prepared - altough it is little
known in literature.
Btw in "Ignition of Explosives by radiation" ammonium tetraperoxychromate is mentioned as a "red-brown" powder with a detonation temperature of 90°C.

Stability might have to do with water of hydration. Hydrated peroxychromates such as Li3[Cr(O2)4]*10H2O, Na3[Cr(O2)4]*14H2O, and Cs3[Cr(O2)4]*3H2O are
known. The hydrated potassium salt is said to loose water of crystalliation if it is washed with ethanol or ether (I'm quite sure that acetone also
works). The water stored in the crystal lattice might be detrimental to stability if it is lost easily and then accumulates to take some of the
compound into solution where it decomposes. That might be the reason why the "wet peroxychromates" are said to be so unstable.
So to summarize:
-A newer dissertation from 2003 proves that the ammonium salt does exist and is isomorph to K3CrO8 (no ammine!)
-It is not yet proven that the ammonium salt is unstable at room temperature. Overanxious handling of other peroxychromates, such as storing K3CrO8 at
freezing temperatures altough its not necessary, is commonly found in literature
-Water of crystallization might be a major factor in decomposition. Thorough washing with ethanol/ether seems to be a very good idea!
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
I have done some calculations on the [Cr(O2)2(NH3)3] synthesis given in Brauer. It uses 3,75g CrO3, 25ml 10% ammonia and 5ml 30% H2O2. There is a
striking shortage of H2O2!
Considering that the reaction should go something like
CrO4(2-) + 3H2O2 + 2OH(-) + 3NH3 ---> [Cr(O2)2(NH3)3] + 2H2O + O2 + 2O(2-)
(the 2O(2-) would be protonated to 2H2O in aequous solution)
there should be 11,6ml of H2O2 - twice as much as used in Brauers synthesis!
Now FINALLY something falls into place  This is consistent with the ERNSTH .
RIESENFELD statement I cited earlier, stating that the triammine complex is formed only if This is consistent with the ERNSTH .
RIESENFELD statement I cited earlier, stating that the triammine complex is formed only if
1. the temperature is allowed to rise above 0°C
and/or
2. insufficient peroxide is used (!)
otherwise, ammonium perchromate is said to be formed.
Given that the Brauer synthesis settles for such a shitty yield as 0,3g of the triammine complex from 4g of CrO3 and goes through the hassle of
filtering out unreacted (NH4)2CrO4, we now have a strong hint that the peroxide shortage is essential for the formation of [Cr(O2)2(NH3)3]!
This might be due to NH3 and O-O competing for ligand places around the Cr(+IV). A shortage of H2O2 likely prevents formation of peroxychromate.
Also, temperatures well above 0°C destroy any peroxychromate and leave the triammine complex only, which is said to be stable in strong ammonia
water. In one paper they replaced the NH3 by CN by heating the triammine in ammoniacal solution with KCN for 5 hours, so dont't be shy to turn up the
heat! 
-----
Summary:
-The triammine complex is much more stable at elevated temperatures than CrO8(3-)
-Formation of the triammine complex is favored by shortage of H2O2
-Any peroxychromate can be turned into the triammine by heating with ammonia water to 50°C
If the above is correct, [Cr(NH3)3(O2)2] can be made conveniently from potassium dichromate and H2O2! No need to use fancy ammonium dichromate which
is hard to come by. Reaction with potassium dichromate would certainly go though an peroxychromate intermediate which can be restrained/destroyed by
shortage of H2O2 and heating. 
Will try that soon. Btw Diperoxoaquoethylenediaminechromium(IV) also sounds interesting, but is said to change color within a few days at room
temperature. It explodes violently when heated.
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
Please refrain from posting off-topic crap.
[Edited on 27-3-2008 by vulture]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Taoiseach, thanks for all the info you have given me, over here, and via private communication. I start to see some pattern in this.
Summarizing it all, I have the impression that there are 4 classes of peroxo chromium compounds:
1) The dark red/brown peroxo chromates, based on the [Cr(O2)4](3-) ion with chromium in +5 oxidation state.
2) The neutral diperoxo species, based on a [Cr(O2)2](2+) core, with other ligands added, with chromium in the +6 oxidation state. Most common is the
deep blue Cr(O2)2O(OH2) (a.k.a. hydrated CrO5), but at somewhat higher pH there can also be violet [Cr(O2)O(OH)](-). I have seen them both, but could
not isolate them.
3) Another neutral peroxo species, based on a Cr(O2)2 core, with other ligands added, with chromium in the +4 oxidation state. An example is
Cr(O2)2(NH3)3, but another example is K3[Cr(O2)2(CN)3]. We made the first example. These are red/brown compounds.
4) Blue dichromate-derived peroxo species, based on a Cr2O12(2-) ion. I have not yet seen these. I'll try to make some of this species also. Whether I
can isolate or not is a matter of trial (and error?).
In older literature many other weird species are mentioned, but I think you are right, that it is better to neglect pre-1980 information about the
peroxo species. Unfortunately, also in newer times, there are quite some people who do not really investigate the compounds and assign properties to
them which are not true. I have a 17 year old sample of K3CrO8 and it is still as explosive and energetic as when I made it.
[Edited on 27-3-08 by woelen]
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
woelen, I have pyridine and could try to isoate the pyridine-Cr(O2)2O abduct. I have not found any information on the properties other that it is
explosive. How toxic do you think it is? I have deep respect for organo-metallic compounds because many are exceedingly toxic, especially those with
carcinogenic metals.
It should precipate from a ammonium bichromate solution by adding H2O2 and pyridine, according to Hiendelmaier this even works in dilute solution.
Also there is a synthesis you could try to make blue ammonium perchromate quite easily (I have not tried yet): Add 4ccm saturated ammonium bichromate
solution to 3ccm of a saturated ammonium nitrate solution. Cool to 0°C. Filtrate, then add 4ccm 30% H2O2 and cool below 0°C. Dark-blue ammonium
peroxochromate will precipate. This is from a 1905 paper by Hofmann and Hiendlmaier , they had given it the formula
NH4CrO5+H2O2
but really it probably is NH4[Cr(O2)2O(OH)]*H2O, i.e. the neutral Cr(VI) species you mentioned with one OH- that gives the negative charge.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Taoiseach
woelen, I have pyridine and could try to isoate the pyridine-Cr(O2)2O abduct. I have not found any information on the properties other that it is
explosive. |
Gmelin's Handbuch (on p. 415, Chrom, Teil C) describes a bit about CrO5.pyridine: in a very dry condition it is stable for weeks, in a somewhat moist
condition it decomposes eventually to CrO3 and a brown resin-like substance. Dry heating under already below 100º causes explosive decomposition. And
from time to time, it explodes even without heating, also from friction, or on contact with conc. H2SO4 or concd. alkaline solutions.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Taoiseach, I think that the pyridine-CrO5 adduct is not more toxic than the individual compounds, so I consider it as toxic as the most toxic one of
CrO5 and pyridine. I'm not sure about pyridine's toxicity (I don't have any of this).
I tried to make the blue peroxochromate, K2Cr2O12. This miserably failed. I had a very nasty and violent runaway. I mixed a saturated solution of
K2Cr2O7 (ice cold, from the fridge at -18 C, frozen) and H2O2 30% (also ice cold from the fridge, but still liquid). When the liquids were mixed, then
the ice quickly melted and the liquid became very dark blue/purple. Within a few seconds, suddenly the liquid became much warmer and it seemed that at
once all H2O2 decomposed. The dark liquid was spewed out of the test tube. Luckily this did not happen inside the freezer, I just had enough time to
dump all of it in the sink.
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Bad news :(
My ammonium perchromate has decomposed. I had put a little sample of both the potassium and ammonium salt on a sheet of paper and left it in open air.
The potassium salt is unchanged and still explosive. The ammonium salt however turned into yellow junk that doesn't explode anymore. I figure that
most of it was oxidized to ammonium chromate.
The bulk of my ammonium perchromate was stored in an airtight PET bottle. I noticed that it was pressurized and had large dents. I was very anxious to
open it, not knowing how explosive the decomposition product might be or if it would be set off by pressure. So I wore ear protection, safety googles
and thick leather gloves, also not directly touching the bottle but rather unscrewing it with a pipe wrench. The gas escaped with a loud hissss sound
and luckily, nothing exploded.
The stuff inside the bottle had not changed much visually (it seems a bit lighter in color), but it now burns MUCH faster. Not as instantaneously as
the [Cr(NH3)3(O2)2] does, but like black powder. It seems it was partially converted into the triammine. When mixed with fine iron powder, its
explosive properties were lame, giving a big yellow flame but only a low report - nothing compared to the incredibly powerful explosion I got from
the fresh perchromate.
I destroyed the rest of it because storing a decomposing explosive would be too dangerous.
So to recap: Ammonium perchromate is not stable at room temperature, probably decomposing into chromate and the triammine complex.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
| Quote: | Originally posted by woelen
it is better to neglect pre-1980 information about the peroxo species. |
http://dx.doi.org/10.1039/JR9620003948
Neglect away. It is easy for me to neglect this, but this is based on not having electronic RSC access.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Here's what S.C. Wack was referring to. They seem to have done quite a lot of redox titrations.
If one isn't too scared of toxicity, the thallium(I) salt is purported by the paper to be especially sensitive.
sparky (~_~)
Attachment: peroxchrom.pdf (633kB)
This file has been downloaded 1105 times
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I can only say WOW!
I did a hammer test with the K3CrO8 and with a mix of K3CrO8 and sulphur.
Pure K3CrO8 could easily be decomposed, by simply hitting with a hammer. No hard banging was needed. The decomposition was accompanied with formation
of some smoke and some noise. Not really impressive, but it was very instructive to see how easily I could get it decomposed, simply by hitting with a
hammer. I did the test with some of the solid, wrapped in a small piece of Al-foil.
Next, I did a test with K3CrO8 + S. No measured amounts, just mixing appr. 2 parts of K3CrO8 (coarsely crushed crystals) with 1 parts of flowers of
sulphur, amounts estimated by the eye. Again, wrapped in Al-foil and carefully tapped with a hammer, on a concrete tile. The result was really
impressive  , given the small amount used for this experiment. There was a very
loud bang, at first hit! , given the small amount used for this experiment. There was a very
loud bang, at first hit!
I can confirm the results of Taoiseach, the material is very energetic, and also impact sensitive, more so, than I expected.
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Yes woelen it needs little energy to decompose, even without fuel. I got scared when I realized how easily it goes off - I had made way too much of
this stuff, in retrospect.
NHCrO8 + Fe was even more impressive. I'm not impressed easily as I've synthesized a bunch of primaries and tested them before. It was incredibly loud
and gave a very long-lasting shockwave. Its a pitty this stuff decomposes.
Anyone dare to try hydrazine perchromate?  
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Taoiseach Anyone dare to try hydrazine perchromate?   |
Fun for the whole family, right? 
No mention of the hydrazine but here's a rundown of the idea of explosibility of some other peroxychromates (from Gmelin Cr [B] p. 750-2 and Cr [C]
410-8).
trimethylammonium peroxochromate, black solid, when the apparently very pure substance was on a scale to be analyzed until the very
small amount exploded with a deafening bang.
tetramethylammonium peroxochromate. [(CH3)4N].CrO5. brown-violet solid, hisses from strong heating giving off O2 and forming a bright
yellow powder, which if heated further burns giving off sparks. Alkali hydroxides, acids, BaCl2- or AgNO3- solution decompose it.
piperidinium peroxochromate (C5H12N)CrO5. in direct light black, and partial light violet solid (from cold dark brown oil). Pretty
stable when in the cold. Upon heating creates a bright explosion.
anilinium (C6H8N)CrO5. After its preparation, as it is being dried shortly on clay, and then KOH for 4 hours in the desiccator, it is
recommended to surround this with ice, since the compound explodes far more violently than the pyridinium compound. The compound decomposes in 1 to 2
days, decomposes by heating and with conc. H2SO4 or alkalies under explosion. With dilute acids or alkali hydroxides releases O2.
chinolium.CrO5. little crystaline plates, not stable for long. Decomposes from heat with the evolution of light and forming chromium
oxide.
guanidinium peroxochromate (CN3H6)3CrO8.H2O. yellow-brown powder. In the dry state can be stored for several weeks. The compound is
not explosive, does not ignite when grinded in a porcelain bowl, but hisses when heated giving off white smoke.
1,10-phenanthroline.CrO5: light-blue powder. Stable at room temperature. Non-explosive. Conc. H2SO4 liberates O2 and forms Cr2(SO4)3.
With boiling alkali solution liberates O2 and forms chromate.
ammonium peroxochromates: (NH4)2CrO5, (NH4)3CrO8, and (NH4)2Cr2O12.2H2O have been identified.
(NH4)2CrO5 is very unstable and the brown solid will ignite spontaneously a little above room temperature, with H2O it forms a yellow
orange solution.
(NH4)3CrO8, small red-brown, octahedral crystals with a reddish glance. This compound must be analyzed in the wet condition, since
when completley dry it decomposes. It is storeable for a longer period in an atmosphere saturated with H2O-vapors. At about 40 deg.C. it converts to
the chromate, at 50 deg.C. it decomposes explosively forming chromium oxide. Pouring conc. H2SO4 over the salt gives a violent reaction with the
evolution of light and forms Cr2O3 which vaporizes in green flakes. The same reaction results when bumped or shocked but here is a strong detonation.
It is decomposed noticeably from H2O even at 0 degrees.
(NH4)2Cr2O12.2H2O: violet-black powder. The dry compound is storeable in the dry state for a few days, but in air decomposes within
24 h completley to (NH4)2Cr2O7. Burns with a loud hiss when heated strongly, forming chromium oxide and red-brown nitrogen oxides vapors. The conc.
aq. solution decomposes pretty quickly to (NH4)2Cr2O7 and liberating oxygen.
[Edited on 10-4-2008 by Hammerl]
|
|
|
-jeffB
Hazard to Others
  
Posts: 185
Registered: 6-12-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Hammerl
chinolium.CrO5. little crystaline plates, not stable for long. Decomposes from heat with the evolution of light and forming chromium
oxide.
|
Did you mean cholinium?
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by -jeffB
| Quote: | Originally posted by Hammerl
chinolium.CrO5. little crystaline plates, not stable for long. Decomposes from heat with the evolution of light and forming chromium
oxide.
|
Did you mean cholinium? |
I didn't know the Latin name would be much different in German as English, but you would know it as quinolinium (C9H7N), a.k.a. chinoline, quinoline,
etc. I did leave out an in it should be "quinolinium", and the given formula for the compound is (C9H8N)CrO5.
[Edited on 13-4-2008 by Hammerl]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I tried to make the barium salt of CrO8(3-), but this does not work.
I have tried the following: Dissolve some K3CrO8 in a solution of NaOH. The reason of doing this is that dissolving in plain water results in visible
decomposition (very small bubbles of oxygen appear). In a solution of NaOH this does not occur. Problem I have, is that K3CrO8 is only VERY sparingly
soluble. In a test tube, half filled with a solution of NaOH, I only can dissolve a tiny spatula of solid K3CrO8. Heating does not work, if I do that,
then I again see bubbles of oxygen.
In another test tube I dissolved Ba(OH)2.8H2O. Next, I mixed the two liquids. This results in formation of a yellow/brown precipitate, which is
amazingly hard to separate from the liquid. It is very flocculent and hardly settles.
I used pre-cooked water, in order to get rid of carbon dioxide (otherwise a precipitate of BaCO3 is formed as well) and flooded both test tubes with
butane gas (my cheap-ass method of creating an 'inert' atmosphere  ). ).
So, I did get some brown precipitate (actually quite light, yellow brown), but the amount was very small, and it was a pain to isolate. Because of the
very small amount, I could not separate it from the filter paper, it was just a thin layer on the filter paper, half-way inside the paper.
After a lot of hassle I gave up. Probably, the only method of isolating this stuff is using a centrifuge, but I don't have one.
The main problem is the very poor solubility of K3CrO8. I see no other option for making the barium salt. Direct reaction with barium salts does not
work, because of the very low solubility of barium chromate. Working wit Ba(OH)2 and CrO3 or working with a sodium chromate solution and adding barium
hydroxide to that do not work.
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Check out the pic - this is what I obtained by adding BaCl2 to a solution of ammonium perchromate (which must have been at least partly decomposed
already at that time). A slimy flocculent precipate appeared that did not look exactly like the bright-yellow dust-like precipate formed by addition
of Ba2+ to a solution containing CrO4(2-). Maybe a mixture of barium chromate and barium perchromate? The stuff was so light and fluffy it could not
be filtered.
[Edited on 17-4-2008 by Taoiseach]

|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I now did an experiment with the CrO5-pyridine complex. I did not isolate this complex, it is terrible, due to formation of a foam. But the experiment
is very nice on its own with surprising color combinations in a single test tube.
http://woelen.homescience.net/science/chem/exps/cr_peroxo_py...
EDIT(woelen): Modified link, such that it works again
[Edited on 8-7-13 by woelen]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Those are awesome pictures. But that's strange it is a foam, it is said to be glancing platelets or needles. Could it be decomposition? The references
say even while drying it in the dessicator it should be in ice because it deflagrates at low temperatures already. The reports on stability are a
lightly conflicting where one says it stable for weeks when totally dry, another notes occasional spontaneous explosion. But the reaction with conc.
H2SO4 or conc. alkali is real conflicting, where one says no explosion and another does say interaction causes explosion. Below are the full sources.
I've even found a Beilstein entry.
Gmelin I.
Gmelin II.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I indeed think it is due to (slow) decomposition. Probably very small particles are formed, which slowly produce oxygen. This causes the particles to
go upwards and stick to the surface of the liquid.
Another thing is that the complex is made in aqueous solution and not extracted into an organic solvent. This experiment was not with the intent to
isolate the complex, just to see how it looks like and what its properties are.
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Here's by far the most comprehensive treatise on peroxo metallates:
http://ir.lib.sfu.ca/bitstream/1892/5801/1/b13595143.pdf
Interestingly the author reports two tetraperoxo complexes of chromium -a trivalent CrV complex made in alkaline solution upon addition of H2O2 to
CrO4(2-), and a divalent CrVI complex made in strongly alkaline solution at low temperature from CrO4(2-) and H2O2. It is only due to variations in
temperature and OH- concentration that two entirley different compounds are formed!
It seems quite possible that the black stuff woelen prepared and the red stuff I got are not just crystal modifications but entirely different
compounds. The red stuff was precipated at much lower temperatures and could well be K2Cr(O2)4. The brown stuff from one of woelens earlier batches
might be a mixture of of the CrVI and CrV complex.
[Edited on 21-11-2008 by Taoiseach]
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
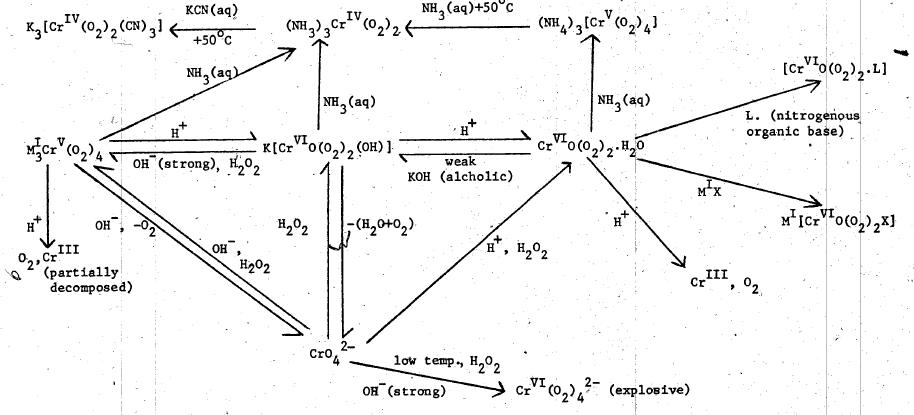
Check out attachment
This is the most interesting part. Check out the lower left part of the graph, starting from CrO4((2-)
|
|
|
| Pages:
1
2
3
4 |