ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
Interesting Reaction Results
Recently I've developed a curiosity about the composition of various hardware store items, and so have subjected them to several methods of inorganic
assaying--sometimes with very peculiar results.
First: A standard bright finish steel nail subjected to pirhana solution.
It is my understanding that the term 'bright' applied to a nail means that it has not been coated or treated with any anti-corrosives, and that it is
simply the exposed steel. Upon completion of the reaction, I was left with a milky white precipitate extraordinarily similar to MgOH.
The H2O2 was 27%, the Sulfuric Acid was 94%. Other results on these nails were more expected: H2O2+NaCl yeilded iron oxide, H2O2+HCl yielded iron
chloride, etc.
Any thoughts on what the milky white compound might be would be appreciated.
(More results to follow.)
[Edited on 4-25-2008 by ShadowWarrior4444]
[Edited on 4-25-2008 by ShadowWarrior4444]
[Edited on 4-26-2008 by ShadowWarrior4444]
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
Recently (several minutes ago) I attempted to produce phosphoric acid from simple garden phosphate (Triple Superphospate, or Monocalcium Phosphate,
indicated as equivalent to 46% P2O5) with some interesting results. I used the Sulfuric Acid method, (oxalic acid will be next.)
The phosphate was in the form small granules which I did not powder. Upon addition of conc. sulfuric acid, a white layer form on the outside of the
granules (which I take to be calcium sulfate,) however, bubbling did occur. I waited a bit, then proceeded to dilute this sample with water (yes yes,
never add water to acid *was quite careful.*) The borosilicate culture tube heated up as expected. Two layers then proceeded to form, the bottom
appeared to contain the brown granular form of the phosphate along with something resembling sulfuric acid. Fairly strange results followed after
this--a purple haze developed and traveled slightly up into the aqueous layer, a while later, a yellow layer developed beneath the purple. No scents
were noticed.
I then decided, largely because I'm a piranha solution fanboi, to employ it again here. The phosphate was added and vigorous bubbling was observed--I
immediately cooled the tube in cold water bath (always on hand) and waited as the tube filled with yellow lather. The tube smelled very strongly of
ozone.
[Edited on 4-26-2008 by ShadowWarrior4444]
[Edited on 4-26-2008 by ShadowWarrior4444]
|
|
|
CyrusGrey
Hazard to Others
  
Posts: 123
Registered: 20-1-2007
Location: USA
Member Is Offline
Mood: Oooh! Shiny!
|
|
Not sure what is going on with your nail. If its stainless steel it will contain chromium and carbon along with the iron, and some kinds contain
nickel or manganese.
Your garden phosphate is probably chock full of impurities. The bubbles might be from carbonates in with the phosphates. The colors could be a number
of things, but I'm no expert. It seems that every fertilizer I have found smells 'fertilizery' and I'm not sure what is causing this smell.
If your interested in using hardware store chemicals as reagents then you might check out the website in my signature. Among other things, we are
putting together a list of over the counter chemicals that are useful to hobby chemistry:
http://www.homechemistry.org/index.php?title=Over_The_Counte...
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by CyrusGrey
Not sure what is going on with your nail. If its stainless steel it will contain chromium and carbon along with the iron, and some kinds contain
nickel or manganese.
Your garden phosphate is probably chock full of impurities. The bubbles might be from carbonates in with the phosphates. The colors could be a number
of things, but I'm no expert. It seems that every fertilizer I have found smells 'fertilizery' and I'm not sure what is causing this smell.
If your interested in using hardware store chemicals as reagents then you might check out the website in my signature. Among other things, we are
putting together a list of over the counter chemicals that are useful to hobby chemistry:
http://www.homechemistry.org/index.php?title=Over_The_Counte... |
The nail is not stainless, and under strongly oxidizing conditions I would have expected to see visible signs of manganese, although the amount used
to alloy steel is usually quite low. It is intriguing that despite the likelihood of having many metals alloyed in (perhaps even molybdenum,) only one
uniform white precipitate is observed. Is iron capable of forming a hydroxide or oxide that is not the charachterisitc red or brown? The only other
possibility would seem to be an iron-sulfur compound. This may be the most likely one, however it is certainly not a sulfate or sulfite. Could Iron
have formed a sulfur compound in a higher-than-normal oxidation state?
Edit: Or! Now that I think of it, there have been theories that life formed above geothermal vents initially through Iron-Sulfur compounds. As there
was carbon in the steel, perhaps an iron-sulfur-carbon compound.
The fertilizer did initially smell 'fertilizery,' this may be due to the presence of carbonates, that may also explain the bubbling as well--the most
curious occurrence was the smell of ozone, it may be that the ozone is being generated by the conjunction of phosphate peroxide and sulfate. The other
possibility is that I am smelling highly concentrated phosphoric acid, the scents are similar in that phosphoric acid smells a wee bit like Sprite.
On another note, this website may help you put together the OTC reagent list more expediently: http://www.hyperdeath.co.uk/chemicals/index.php
[Edited on 4-26-2008 by ShadowWarrior4444]
[Edited on 4-26-2008 by ShadowWarrior4444]
[Edited on 4-26-2008 by ShadowWarrior4444]
[Edited on 4-26-2008 by ShadowWarrior4444]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Bright nails are zinc electroplated. The white should then be Zn(OH)2, which is indeed similar to Mg(OH)2. Why you observed it precipitating in
acidic solution is beyond me, unless you meant to rephrase that.
Tim
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by 12AX7
Bright nails are zinc electroplated. The white should then be Zn(OH)2, which is indeed similar to Mg(OH)2. Why you observed it precipitating in
acidic solution is beyond me, unless you meant to rephrase that.
Tim |
They cannot have been zinc electroplated-- the terminology for any nail that has a zinc coating is usually "Galvanized." Moreover, the nail has no
immediate and vigorous reaction to HCl or to KOH, indicating that there can be no zinc present on the surface. This was tested against a nail I knew
to be galvanized with zinc, and a screw listed as a "Zinc Screw."
All other chemical tests thus far have indicated only the overwhelming presence of Iron, with no other metal ions showing visibly. Perhaps if the nail
was alloyed with zinc or magnesium... however the solution was indeed *very* acidic. H2O2 has been known to act as a weak base, though I don't see how
this could in any way form a hydroxide--especially with all the protons flying around.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Alright, then how about.... silica? There's only a very small amount of silicon in steel.
Repeat your reaction under more controlled circumstances. If the solution was still acidic, then I don't know HOW you can possibly comprehend the
precipitate as resembling Mg(OH)2, which reacts readily with acid!
Tim
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
An Update: The piranha/phosphate vial has turned a deep black, with a freely flowing grey powder at the bottom. Some brown powdery substance is
flowing through the black. These are both likely impurities, perhaps ancillary rocks mixed in with the phosphate rock used in the process. The tube
smells slightly of phosphoric acid with an irritating overtone reminiscent of HCl. The vapor seems to predominantly irritate mucosal membranes, and is
only present quite close to the tube lip--as it does not travel far from the tube, it may be more dense than air.
Something New: I came across a plumbing solder some time that is listed as containing Tin, Copper, and Selenium. A little research indicated that it
is mostly tin (>98%), with copper being a standard addition for stability. The selenium is apparently to prevent oxidation.
Exposed to 38% HCl, it apparently does not form tin chloride; instead with moderate bubbling of hydrogen it appears to alter its form to a grey
powder. Grey powders are popular when working with tin, and as such I'm not entirely sure *which* one this is. It most closely resembles tin's
nonmetallic allotrope. No copper or selenium chlorides were observed. I will evaporate the water at some point to look for any crystallization of
SnCl2.
More interesting were the products of exposure to H2O2/HCl:
At the completion of the reaction, 3 layers had formed--the top layer appeared the most aqueous, and had the characteristic sky/light-blue tint of
copper. Beneath this was a layer of bright yellow which I suspect is selenium chloride, and under that is the irrepressible grey powder.
In an earlier experiment under the same conditions only in a small beaker, the blue has settled out leaving only a light canary-yellow aqueous
solution. I recrystallized this in a Petri dish yielding very thin similarly colored translucent fractally oriented crystals. At this point there was
an enjoyably grey powder left on the bottom of the beaker, to which I added a bit more H2O2. The result was a vigorous exotherm followed by the
appearance of a light-blue solution.
[Edited on 4-26-2008 by ShadowWarrior4444]
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by 12AX7
Alright, then how about.... silica? There's only a very small amount of silicon in steel.
Repeat your reaction under more controlled circumstances. If the solution was still acidic, then I don't know HOW you can possibly comprehend the
precipitate as resembling Mg(OH)2, which reacts readily with acid!
Tim |
I should clarify: 'resemble' in sense of looking exactly like MgOH, I am fairly certain that it does not have any of the chemical properties
associated with MgOH--I was merely referring to its appearance. Upon examination again today, it is not quite as white as MgOH, the precipitate has
yellowed slightly, and settled to the bottom of the culture tube. The yellowing may be due to minute amounts of iron hydroxide or oxides.
I shall repeat the experiment under the same conditions in another culture tube, however the mix of H2O2/H2SO4 was fairly straightforward.
[Edited on 4-26-2008 by ShadowWarrior4444]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
From Wikipedia:
Bright - no surface coating; not recommended for weather exposure or acidic or treated lumber
However this does not mean that the nail itself is not an alloy of steel. Zinc could well be present, if alloyed then it would react slower than a
galvanised nail or a zinc screw.
You could also try performing a flame test on the nail. Zinc colours the flame blue-green, and there should be a fair bit of gold colouration due to
the iron content of the nail 
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Nah, I don't know of any steel alloys that actually contain zinc (according to the phase diagram, it could happen, being fully soluble, probably
having a strengthening / embrittling effect as with many elements, but as far as I know it isn't used).
Tim
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Run of the mill nails are generally made with mild steel, which is at the higher carbon content end of the low carbon steels - 0,15 to 0,3 percent
carbon. It may also contain up to a percent or so of manganese, a half percent of so of silicon, and possibly a fraction of a percent of copper.
Other metals will be in rather small fractional percentages if at all.
Bright does mean just the bare steel, no intentional coating applied. Don't be surprised to find a trace of oil on them, though.
The Sn/Cu/(Se,Te) solders generally have between 3 to 6 percent copper, centered in the 4 to 5 percent range. Selenium or tellurium is in the range
of 0,1 to 0,3 percent, below that it doesn't enhance the melting and above that the solder tends to get 'runny'. That amount of Se/Te doesn't seem
likely to give more than a film, not a distinct layer. If you're using tech grade ('hardware store') HCl I'd be inclined to blame yellow colouration
on iron in the acid.
It is possible that the solder is crystals of one metal in a matrix of another, with most of the alloying metals ending up in one phase or the other.
Treatment with acid could preferentially dissolve one phase leaving the other as a powder, which would appear grey from its fine subdivision. Wash it
free from acid, rinse with alcohol and then acetone, dry, then press between hard non-metallic surfaces (even two rounded rocks) and see if you can't
form a metallic film.
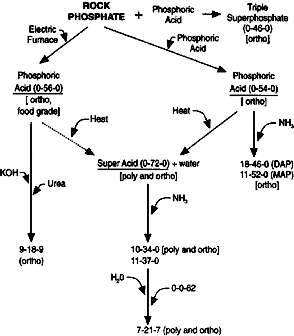
Agricultural or even horticultural grade phosphates are likely to be a mess. Some calcium, some sulphate, traces of fluoride, iron, aluminium, and
silicon; depending on how honest the manufacture trace amount of other heavy metals show up as well. If you don't make it into a paste with a little
water before adding acid, expect bubbles from trapped air and from the reaction between surface and complexed water and the concentrated H2SO4.
There's at least a half dozen simple compounds that smell "like ozone", most people's noses are not very good at telling the difference. If you don't
actually run tests to distinguish between them don't assume ozone.
The actual volume of something suspended in a liquid can often be deceptive. Isolating and weighting is the way to go to see if what you have is a
significant component or just a trace product. Even filtering and washing can help, although some precipitates can be rather bulky and hold a lot of
water.
[Edited on 27-4-2008 by not_important]
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by not_important
Run of the mill nails are generally made with mild steel, which is at the higher carbon content end of the low carbon steels - 0,15 to 0,3 percent
carbon. It may also contain up to a percent or so of manganese, a half percent of so of silicon, and possibly a fraction of a percent of copper.
Other metals will be in rather small fractional percentages if at all.
Bright does mean just the bare steel, no intentional coating applied. Don't be surprised to find a trace of oil on them, though.
The Sn/Cu/(Se,Te) solders generally have between 3 to 6 percent copper, centered in the 4 to 5 percent range. Selenium or tellurium is in the range
of 0,1 to 0,3 percent, below that it doesn't enhance the melting and above that the solder tends to get 'runny'. That amount of Se/Te doesn't seem
likely to give more than a film, not a distinct layer. If you're using tech grade ('hardware store') HCl I'd be inclined to blame yellow colouration
on iron in the acid.
It is possible that the solder is crystals of one metal in a matrix of another, with most of the alloying metals ending up in one phase or the other.
Treatment with acid could preferentially dissolve one phase leaving the other as a powder, which would appear grey from its fine subdivision. Wash it
free from acid, rinse with alcohol and then acetone, dry, then press between hard non-metallic surfaces (even two rounded rocks) and see if you can't
form a metallic film.
Agricultural or even horticultural grade phosphates are likely to be a mess. Some calcium, some sulphate, traces of fluoride, iron, aluminium, and
silicon; depending on how honest the manufacture trace amount of other heavy metals show up as well. If you don't make it into a paste with a little
water before adding acid, expect bubbles from trapped air and from the reaction between surface and complexed water and the concentrated H2SO4.
There's at least a half dozen simple compounds that smell "like ozone", most people's noses are not very good at telling the difference. If you don't
actually run tests to distinguish between them don't assume ozone.
The actual volume of something suspended in a liquid can often be deceptive. Isolating and weighting is the way to go to see if what you have is a
significant component or just a trace product. Even filtering and washing can help, although some precipitates can be rather bulky and hold a lot of
water.
[Edited on 27-4-2008 by not_important] |
I was under the impression that the yellow coloring of technical grade HCl was due to excess dissolved chlorine. In any case, I am fairly certain that
the appearance of a yellow layer is not caused by this original coloring--when the solder is dissolved in only HCl no yellow is apparent, only a slow
breakdown of the solder into the aforementioned grey powder accompanied by a bit of bubbling. A yellow layer only becomes clearly visible upon
addition of H2O2, and while this may be due to iron chloride, I would expect that to be fully soluble in the water, not settle toward the bottom.
An Update: The sky blue of the copper ions has completely vanished leaving only the yellow layer and a clear water layer. It appears that the copper
may have precipitated out; there is a metallic sheen atop the grey powder that was not there before. A note on the grey powder: It is not a fine
powder. I believe it is the alpha-tin allotrope, though I will attempt to form a metallic film out of it.
If it is alpha-tin, it may be formed by the action of the HCl dissolving the tin in a non-uniform manner. It may also explain the precipitation of
copper onto the tin--a displacement reaction. Another possible explanation for the yellow color/disappearance of blue may be Copper(I) Chloride. The
blue may well be Cu(II) which is then reduced by the alpha-tin to Cu(I), which is yellow in color. Also, based on the crystal formation, it is
increasingly likely that they are white crystals of SnCl2 interspersed with CuCl. The only other possibility is Selenium Chloride, which is described
as an oily yellow liquid.
As for the phosphates, I happened upon an experiment by BromicAcid that had identical results when he was attempting to produce pyro/metaphosphoric
acid. In his work, it was caused by conc sulfuric acid/heat on the fertilizer, in mine the decomp. of H2O2 provided the heat. The results are most
likely due to excessive impurities in the product.
The mystery that particularly interests me is the fluffy-white precipitate from H2SO4/H2O2 and a common nail. I have confirmed that there was no oil
on it, and the volume of precipitate seems to indicate that it is formed from an element that is present in a non-trivial amount. Even more
interesting is that it is the *only* thing that formed in an appreciable quantity. In such strongly oxidizing conditions, one might expect a bit of
iron oxide, or even iron sulfate (as iron chloride is formed from HCl and H2O2.)
My next step will be attempting to form a larger quantity of this substance and subjecting it to a more rigorous analysis. Side notes: Its formation
is accompanied by moderate bubbling of a gas which I shall collect, and it has remained stable and insoluble in water having the consistency and
coloration of flour.
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
Update: The flour-like substance has the scent of H2O2, and now that I think of various other posibilities, it may be that the H2SO4 and H2O2 have
formed something akin to potassium monopersulfate (oxone.) Does this compound have a transition metal analog, perhaps iron monopersulfate?
Scaling up the reaction has proved a bit difficult, with the whole Piranha/violent exotherm component. Though, so far the compound seems to only form
when the reaction is done in this order: Nail into tube, H2SO4 into tube, H2O2 into tube.
I'm sure I can smell Oxone! Or maybe Ozone... *slowly drifts into madness.* Though, since I’ve recently generated ozone for comparison, I can say
that this smells quite a bit like it, much more so than the phosphoric vial did.
Upon attempting to scale up a bit further in another vial, the exotherm was a bit more violent, and I believe I detected the scent of H2S. Yellow
frothing/vapor was apparent.
The compound seems to form exclusively at the border between the layer of H2O2 and H2SO4. It may also require a certain temperature--the previous
frothing vial was placed in an Ice/Water bath out of necessity, and though a similar pattern of bubbling is observed, the milky white of the compound
is not present. (Yet.) *watches intently*
It has shown up! Exactly at the border, and slightly upward into the Aq layer. It is forming much more slowly in the cold, giving me a clear view on
just where it forms.
[Edited on 4-27-2008 by ShadowWarrior4444]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
A minute amount of H2S (or given the H2O2, oxidized sulfur species -- "gunpowder" smell), as well as phosphines (including carbon chains,
surprisingly), is typical of dissolving many metals, due to S and P impurities. The nose is very sensitive to these compounds, parts per billion
level; it's no surprise, then, that parts per thousand or million impurities in the metal can be detected.
Tim
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by 12AX7
A minute amount of H2S (or given the H2O2, oxidized sulfur species -- "gunpowder" smell), as well as phosphines (including carbon chains,
surprisingly), is typical of dissolving many metals, due to S and P impurities. The nose is very sensitive to these compounds, parts per billion
level; it's no surprise, then, that parts per thousand or million impurities in the metal can be detected.
Tim |
Yes, I had suspected this was due to impurities.
In other news, I believe I have a viable theory as to what the white precipitate is. I repeated the experiment with copper yielding the same results,
only with a blue solution. This leads me to believe that I have either solid peroxymonosulfuric acid or peroxydisulfuric acid, it formation from H2O2
and H2SO4 catalyzed by the presence of a transition metal catalyst. A simply melting point test should reveal which one it is.
If it is peroxydisulfuric acid, this process would be a lovely OTC source of persulfates. Though, I suspect it is the former peroxymonosulfuric acid,
'Caro's acid.'
The potassium salt of which is Oxone, which the precipitate looks and smells like at the moment. Still, the process may be useful. Thoughts?
I would also like to encourage others to post interesting/unusual/mysterious reaction results to perhaps serve as inspiration for those who might
visit this thread.
[Edited on 4-27-2008 by ShadowWarrior4444]
|
|
|
|