PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Purification of 1,3,5-trioxane
I bought a bunch of trioxane ($5/kilo  ), but much to my dismay the trioxane,
while being 95% pure, has a blue dye which seems to be soluble in both water and non-polar solvents. ), but much to my dismay the trioxane,
while being 95% pure, has a blue dye which seems to be soluble in both water and non-polar solvents.
Does anyone have any practical experience in removing such a dye, or purifying trioxane in general?
I am planning on crystallizing from water (which is what Purification of Laboratory Chemicals 5th ed. suggests), or if that doesn't work, then purify
by distillation (BP = 114*C).
[Edited on 16-5-2008 by PainKilla]
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
So I have found more specifically what the problem is...
http://www.tpub.com/content/MIL-SPEC/MIL-F/MIL-F-10805D/
are the specifications for the trioxane I bought.
They contain:
Trioxane 95%
Magnesium Stearate (binder) 5%
Methylene Blue as a dye (trace)
So the question is either, how to remove the magnesium stearate, or how to purify the trioxane?
I tried recrystallizing from water but that was extremely messy and it did not help very much as far as attaining a product that wasn't blue (it was a
less pale blue though).
It didn't seem to melt either, so I don't know how viable distillation is.
Edit: It seems that the dye is actually not very soluble (though the solution still discolors a bit) in DCM, in which the trioxane is quite soluble...
I hope thus to extract with DCM and distill... It's a pain what else can you do?
Edit2: Activated charcoal appears to remove some of the dye (from very dark blue solution to baby blue)... Just let it sit for a few minutes.
[Edited on 17-5-2008 by PainKilla]
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
I tried purifying via the following method:
120g of the trioxane bars (containing about 110g of trioxane) was dissolved in 450ml of dichloromethane, activated charcoal added and the solution
stirred for 15 minutes. There was a significant portion of trioxane that did not dissolve. The charcoal/magnesium stearate/undissolved solids were
filtered via Buchner funnel and the dichloromethane distilled until about 80% of the original volume used was collected. The remaining (now darker
blue) solution was chilled in the freezer to precipitate the trioxane. Large, sharp, difficult to break colorless needles soon precipitated and these
were washed with a small amount of cold dichloromethane and some acetone. A small amount of dye remained (but the crystals are basically pure, it's
very very very small traces of pale blue on a few crystals). Good enough for me.
30g were recovered. (~30% recovery #_# )
I kept looking for data regarding trioxane solubility and there is a wealth of data available in ACS journals. I tried dissolving the bars in toluene
and that seemed to work perfectly - no dye dissolved (although the solution did turn a lilac color after a bunch dissolved) so this, and not DCM would
be the ideal solvent (although that's pretty obviously given the horrible recovery).
In short, for future reference, people trying to purify these bars should dissolve a bunch of them in hot toluene, quickly filter out the insoluble
stuff, and then throw in the freezer for a while. This should afford very pure and dye/stearate free crystals.
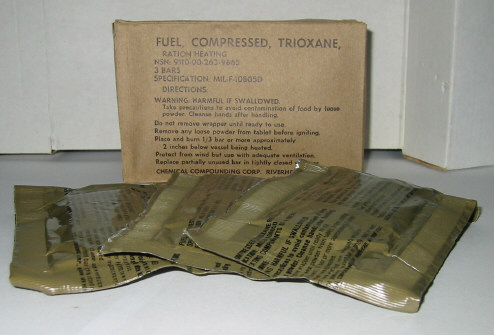
I attached a picture of the bars I'm talking about. I'll probably wind up using the toluene recrystallization before long, so I'll post an update if I
do decide to purify some more trioxane.
[Edited on 18-5-2008 by PainKilla]
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Such a waste of materials! Sublimation is the way to go for 1,3,5 trioxane.
Set a 5 gallon bucket in a waterbath. Melt the trioxane fuel bars in the bottom of the bucket, up to 1/3 full.
Set on heating pad, use snap on lid, come back 1 month later and take out pure trioxane crystals from the top of the bucket,
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Well, I don't need a lot (few grams basically), and I have a bunch of paraformaldehyde so it's not like losing a dollar is going make me go bankrupt.
In any event, I don't have a month to sit and wait, I'd rather have a nice quick procedure.
More importantly, I think using toluene should work fine, especially since neither dye nor magnesium stearate dissolve in it, so one can recycle both
it and the trioxane infinitely (well, expect for the first bit that dissolves).
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Well then just use a wide mouth mason jar, upside down with a empty 8 oz pimento or babyfood glass jar inside as a sublimator. Cheap coffee warmer
hotplate works well. Maybe a day to get 100 grams really pure trioxane.
|
|
|