| Pages:
1
2 |
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Regarding the use of conc H2SO4 as drying agent for SO2
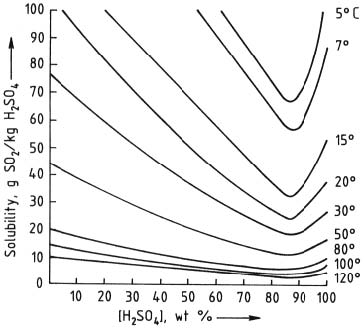
SO2 dissolves in sulfuric acid depending on temperature and concentration of acid. The minima is at c.85% acid by weight. At ordinary (ambient)
temperatures the solubility is about 20 g SO2 (about 300 mmol) per Kg acid. See graph below.
But this nuisance can be overcome by warming the acid after a run which drives off almost all SO2, solubility in 85% acid is almost nil ay 100-120 C.
The SO2 which will be done dry can then be condensed and collected.
[Edited on 31-8-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
According to one of my old books (Handbuch der Pr"aparativen Chemie, written by Ludwig Vanino), a very good method of making dry SO2, allowing a
constant flow of gas, which can be switched off and on, is the following:
- mix 3 parts of calcium sulfite with 1 part of calcium sulfate (gypsum) and make a dry hard solid from this mix, by mixing with some water and
allowing this to harden.
- Make chunks from this hard mix of one to two cm diameter
- Use a Kipps apparatus with sufficient concentrated sulphuric acid for making the sulphur dioxide.
According to Vanino, with 500 grams of CaSO3/CaSO4 chunks one can have a slow but steady stream of SO2 for appr. 30 hours. Higher speed can be
achieved by using a larger Kipp's apparatus, equipped with more CaSO3/CaSO4 mix, or using finer granules. Gas stream can be switched on or off.
The gas, produced from this method already is very dry. If really dry gas is needed, it is suggested to pass the gas through a tube with clean calcium
chloride. The book does not recommend sulphuric acid as drying agent.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks, Woelen, that is interesting. I believe I have Vanino's book in pdf somewhere in my library.
I take it that the calcium sulfate is present as a diluent and binder, obviously it is not participating chemically. The idea must be to limit the
surface area presented to the conc H2SO4 to slow the reaction. Wouldn't the same effect be achieved by adding powdered CaSO3 via a powder addition
funnel (rotary screw feed type?) Also, 500g of such a mixture is not many mols (like, 1.5-2 I'm guessing) of the active sulfite. Generating that
little SO2 in 30 hours is pretty pokey.
Lastly, I know what a Kipp's apparatus is. I have actually seen and handled one in my old high school lab, in the 1960s and it very likely had been
there since the Great Depression. They used it to make H2S. I have not seen one on offer from any of the glassware companies in all my years of
collecting and perusing their catalogs. Are they still made in Europe?
Many references do recommend conc H2SO4 for drying SO2 but it is good to know CaCl2 is an option. See Brauer's chapter on SO2Cl2, he used conc H2SO4
to dry tank Cl2 and SO2 seperately before mixing them and passing them through activated carbon (or camphor) to obtain his product. Just as an
example.
I ran across a prep of SO2 from sodium sulfite or bisulfite using 2 M HCl. They dripped the acid onto the dry sulfite, which sounds like foam city to
me (ask klute.)
Sic gorgeamus a los subjectatus nunc.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Sauron, the CaSO4 indeed is not part of the reaction, it just is there to make it possible to make chunks of the solid. It is not a diluent, but the
gypsum can be made into hard tablets or chunks, which is not possible with CaSO3 alone. Careful and regulated addition of powder to the acid may
certainly be an other option.
500 grams of the mix is just over 3 moles of SO2. That is not very much, but the reaction can be scaled up easily and can be interrupted at any time
you wish. Those are nice properties.
A Kipp's apparatus still can be purchased where I live. A new apparatus has a price of approximately EUR 75 for a small one (250 ml spheres) to EUR
150 for a larger one with 1000 ml spheres. I personally do not have such a piece of glassware, but I am considering buying one. Making gases in this
way can be quite attractive, especially easy to make gases like Cl2, CO2, H2S, H2 and NOx, which all can be made from chemicals in the form of larger
chunks.
Making SO2 from 2M acid does not sound like a good thing to me. The acid is too dilute in that case, and certainly with plain sulfite I do not believe
this will give much gaseous SO2.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Equal parts by weight CaSO3 and CaSO4, so 250 g each, right? Calcium sulfite is 120 g/mol so this is 2 mols and a skosh more, and will yield at best 2
mols (128 g ) SO2 in 30 hrs, slightly more than 4 g per hour. Pretty slow. Too slow for liquifying I think.
I may be interested in a large Kipp's at that price.
Thanks
Sic gorgeamus a los subjectatus nunc.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Equal parts by weight CaSO3 and CaSO4, so 250 g each, right? ... |
woelen said
| Quote: | | mix 3 parts of calcium sulfite with 1 part of calcium sulfate |
Interesting to see how all this turns out.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Ah. Obviously if the mix is 75% Ca sulfite and 1/4 Ca sulfate then indeed 500 g of such mix will contain 3 mols SO2 potentially and not 2. It would
produce 6 g SO2/hr for 30 hrs (according to Vanino). So I simply misremembered the Vanino procedure, and ought to have reread woelen's post. Mea
culpa.
However, this does not alter my conclusions. This procedure is too slow for liquifying the SO2.
Aldrich's agent just quoted me c.$150 for mild steel lecture bottle 440 ml, $75 for 900 ml Sure/Pac cylinder, so it seems that Sure/Pac is 4X more
cost effective for liquified gases than LBs. This means SO2, N2O4, NO, NH3, Cl2, which are vapor over liquid and do not develop high pressures. Not
suitable for HCl, HBr, etc which need the LB and aren't going to be liquified in the lab.
We are going round and round about control valves for which Aldrich's prices are absurd. A simple inline LB valve in SS That was $80 13-14 years ago
is now being quoted at $300. I'm glad I did not ask about their regulators.
Sic gorgeamus a los subjectatus nunc.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Sauron, do you really need to liquefy the SO2? If you don't want to keep it for long term storage, then why not use it directly from the Kipp's
apparatus? If at a certain point you don't need more, you simply shut off the gas stream and you can take out the gas at a later time. When the gas is
not needed at all anymore, then you can reuse the Kipp's apparatus for another gas.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The Kipp's is not needed. And no I do not really need to liquify (well, not always) and can often use the SO2 directly.
Sic gorgeamus a los subjectatus nunc.
|
|
|
| Pages:
1
2 |