Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
Spoiled milk turns red
Does anyone know why spoiled milk turns bright red. This seems to happen when old whipping cream or milk are left out at room temperature for a
while. I presume it is due to bacteria but I haven't been able to find any under the microscope when I look at a sample.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I've never seen red. I've seen blue-green, which I suppose is the color of penicilium.
Tim
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
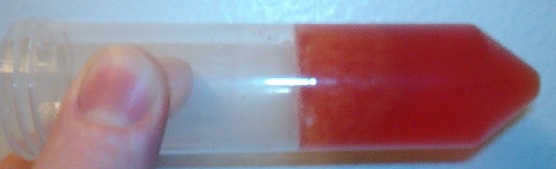
Well here is a picture of the red fluid that I drew off of the spoiled cream. This cream was three months past it's expiration date. It was an off
white rancid and curdy smelling liquid. I let it sit in a milk jug over night at room temp. Then it turned red
[Edited on 22-10-2008 by Trifluoroacetic]
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Omg that is absolutely disgusting  I wouldnt even have dared to open the
container I wouldnt even have dared to open the
container 
I would guess its bacteria/bacterial by products, but I cant find anything on red bacteria in milk.
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Red Yeast?
Perhaps a yeast. Maybe a rhodotorula:
http://www.springerlink.com/content/h4x38727tr147288/
Rhodotorula grown on milk from a yogurt starter is attached.
or a xanthophyllomyces:
http://www.springerlink.com/content/27436320770148l8/
Access to a microscope would be definitive (yeast are usually aggressive and crowd out most other microbes).
Yeast should be obvious, and rhodotorula sp. are ~ 5um in size; see:
http://www.ncyc.co.uk/photo-ncyc-CBS319.html
Cheers,
O3
[Edited on 22-10-2008 by Ozone]
Attachment: red yeast-Frengova-2004.pdf (212kB)
This file has been downloaded 1453 times
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
I've seen red milk mentioned online but nobody states what type of bacteria causes it.
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
I USED THE OIL IMMERSION LENSE on my microscope and i can just barely make out two types of organisms. I see a few rod shaped and round microbes.
I'll culture this tonight on a medium and see if I can get a better picture posted of the microbes up close.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Maybe take a tiny bit and run it on TLC?
Also, the concentration of the red product doesn't need to be very high at all in order to be visible - in other words, it could be just a minor
metabolic side product of i.e tryptophan, cholesterol (and derivatives) oxidation etc.
[Edited on 23-10-2008 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Wow this is really surprising to me! I don't know how much you want to get into this but
I've never seen colored milk like that, Since cream is a lot higher in fat then is other milks that could play an important roll.
Perhaps if you don't have TLC, or other ways of identifying it, you may be able to get different milks with various levels of oils/fats and see if
they turn red at different rates or intensities. Then also you could try to see if its from an amino acid in the protein as suggested by precipitating
the majority of the proteins using an acid, and then neutralizing it while precipitating the salt out (sulfuric acid, and calcium hydroxide?)
I don't really know if those would work, but if I wanted to find out what caused this, I'd attempt what I just mentioned since it is what have
available to me and I think it could help point me in the right direction. I doubt you'd want to get into it that much though.
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
well I have two culture tubes ready to go. I will make a culture of the red spoiled cream and non spoiled cream. I will also try running a TLC. I will
keep you guys/gals posted on the results and post some pictures in a few days after the cultures are done. I'll go start preparing a TLC plate.
You know this all started from me letting spoiled cream spoil even more in an attempt to extract various stinky fatty acids from it.
I plan on running some tests on the spoiled milk so we will see if it's a protein/ oxidation product that is causing the red coloring.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Serratia marcescens as I recall can color whatever it's growing in red.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
I think I might have figured out what is causing badly spoiled milk to turn red. It might not actually be the color of the bacteria but it might be a
result of the break down/hydrolysis of milk fats and casein. This could be caused by certain strains of bacteria though. aparently some types if
bacteria produce an enzyme that break down casein. A simple test can be done by creating a milk peptone auger and then culturing bacteria on it. if
the plate shows signs of clearing around bacteria colonies then they have this enzyme. I am running a culture right now to test for this. I wondered
if it might be possible to re-create this by hydroliyzing milk with a strong based. So I added lye to a milk sample within minutes it begins to turn
yellow just like spoiled milk. It also seems to release an amine gas possibly ammonia. The following picture is that of rapidly hydrolyzed milk it
looks spoiled but it's not.

|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Maybe an assay for thiobarbituric acid reactive substances (TBARS) resulting from lipid autooxidation might also be a good idea. the polymeric junk
resulting therefrom is frequently reddish in appearance (and smelly).
Chemoleo has a good point. The amount of material required to get a color like that might be on the order of ppm, so it might be hard to detect. I am
interested in the outcome of your experiment.
I too have never seen cream this color. What does it smell like? Acetic or butyric (or both)? That might help us to figure out what you've got.
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
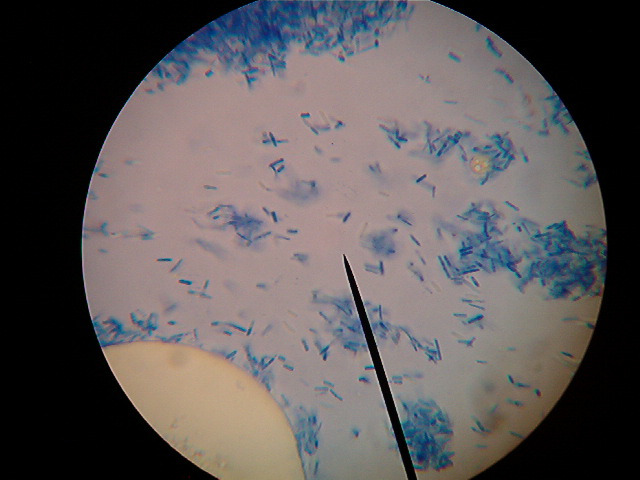
Well I have a slide made of the bacterial culture. The bacteria do appear to be segmented.
[Edited on 26-10-2008 by Trifluoroacetic]
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
If you can, try growing the bacteria on MacConkey agar. I'm really pretty sure it's Serratia
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
I will order some MacConkey ager and run a test. These bacteria seem to have formed a thick plaque in the culture tube at first I didn't think
anything was growing until I noticed the awfull smell so I new they had to be there.
|
|
|