| Pages:
1
2 |
stereochem
Harmless

Posts: 12
Registered: 29-3-2008
Member Is Offline
Mood: No Mood
|
|
Acylation of benzene with anhydrides
I want to make ketone by acylation of benzene with an anhydride and I need a recipe for this reaction. I am interested obvious in short reaction times
and good yields. Can somebody help me?
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
| Quote: | Originally posted by stereochem
...and I need a recipe for this reaction... |
And that's where you went wrong.
You came to the wrong place looking for spoonfeeding. One of your other posts says you're an organic chem student. If you're not full of shit about
that, you should know how to do this.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
harrydrez
Harmless

Posts: 26
Registered: 28-11-2008
Location: usa
Member Is Offline
Mood: content
|
|
Gee I wonder what you want to make there.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
What you want to do is Friedel-Crafts Acylation, closely related to Friedel-Crafts Alkylation. When an aromatic compound reacts with a carboxylic acid
chloride, RC(=O)Cl, in the presence of AlCl3, an acyl group -C(=O)R becomes attached to the ring. Reaction of benzene with, for example, acetyl
chloride, CH3C(=O)Cl, in the presence of AlCl3 at 80ºC, yields (95%) acetophenone, C6H5-C(=O)CH3 . This works for acyl chlorides, but I have been
unable to find any reference to it working with acid anhydrides instead. Because it involves the AlCl4- anion, the latter is most unlikely to work.
[Edited on 7-12-08 by JohnWW]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
JohnWWW- It most certainly works with anhydrides, not to mention other lewis acid catalysts. I imagine these reactions either proceed by protonation
of the carbonyl oxygen in the case of things like sulfuric acid or by formation of a oxyhalide complex for the anhydrous metal halides. The haworth
reaction (actually a multistep synthesis) for example uses succinic anhydride and involves two acylations. fluorescein is synthesized via a friedel
crafts and uses phthalic anhydride with a zinc chloride catalyst, but woelen has shown that conc. sulfuric is acceptable as well.
EDIT: added a possible mechanism with an arbitrary anhydride.
[Edited on 12-7-08 by UnintentionalChaos]
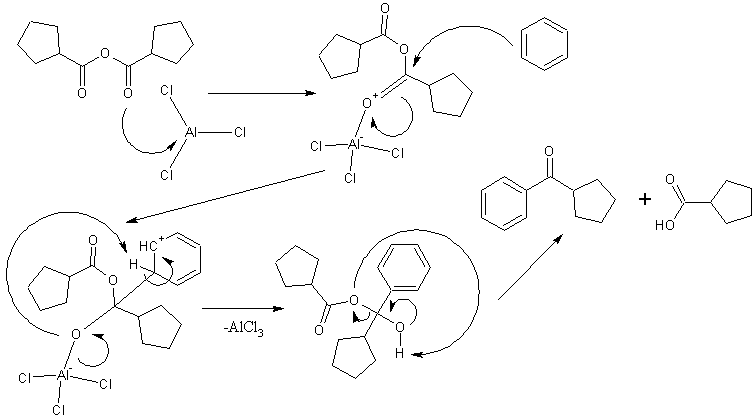
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
stereochem
Harmless

Posts: 12
Registered: 29-3-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by UnintentionalChaos
| Quote: | Originally posted by stereochem
...and I need a recipe for this reaction... |
And that's where you went wrong.
You came to the wrong place looking for spoonfeeding. One of your other posts says you're an organic chem student. If you're not full of shit about
that, you should know how to do this. |
Dear friend, I am an org chem student and I know how to make this acylation in principle, but I need a recipe in order to get a a good yield. I don't
know you make your reactions, maybe a little of this with a little of that, you mix a while and that's it; instead I need a recipe. If you cannot help
then stay at home. And the reaction that I look for is F-C with AlCl3.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
"Recipe" is a word that makes everyone here see red.
Go to the free forum library and download Organic Reactions, volume 1. There is a chapter devoted to F-C acylations, and it undoubtedly covers use of
acid anhydrides. There will be tabular data with references, reaction times and temperatures are usually given, and yields as well. That volume is
rather dated so you might want to go to the ACS search engine at ACS Publications and search Chemical Reviews. The Merck Index section on named
reactions will have a prominent entry of Friedel-Crafts acylation, with references, and there are numerous online surveys on named organic reactions
set up similarly.
But the question remains, if you are as you say, an org.chem student, what year are you in? Because every sophomore not suffering from narcolepsy
knows everything I just wrote above. As S.Holmes used to say - it's elementary.
Sic gorgeamus a los subjectatus nunc.
|
|
|
stereochem
Harmless

Posts: 12
Registered: 29-3-2008
Member Is Offline
Mood: No Mood
|
|
Thank you for your help.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by stereochem
Dear friend, I am an org chem student and I know how to make this acylation in principle, but I need a recipe in order to get a a good yield. I don't
know you make your reactions, maybe a little of this with a little of that, you mix a while and that's it; instead I need a recipe. If you cannot help
then stay at home. And the reaction that I look for is F-C with AlCl3. |
You are a student and know not your way to a library or find scientific papers? And furthermore you ask to be spoonfed with recipes? Ah
well, what can I say... perhaps you should reconsider and study something else?
By the way, when opening new threads in Organic chemistry section you should provide some references and be more specific when describing your
problem/topic. Otherwise please open the thread in Beginnings section if you don't have the time to do a search before posting. I'm moving this thread
there anyway, as it is obviously pedagogic in nature.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thesed kind of posts are really getting me upset. "Give a receipe" isn't going to get you anywhere, especially not in chemistry, and having the
arrogance of telling UnintentionalChaos of staying home, while it should be exactly what you should be doing, is practically hilarious.
You really a student? Learn how to learn before even considering taking any credit from your position...
| Quote: | | ...I don't know you make your reactions, maybe a little of this with a little of that, you mix a while and that's it; instead I need a recipe
|
That description would be one of the closest for the term receipe... So you already have what you asked for.
ANY practical chemistry book would have given you a procedure for a FC acylation, all free ressources from Vogels to OrgSyn.
I'm rather the kind that likes to help begginers, but with such arrogance I stopping right there for you.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
In the US, undergrads are not taught to research, and are spoon-fed all the information they need. If I didn't have an interest in amateur chemistry,
I would have only very basic research skills. Therefore, while I think stereochem IS lazy, I do empathize with him as he is in the same boat as a lot
of undergrad chem students.
It might be handy if someone wrote a brief article in prepublication explaining the basics of chemistry research. Then, in cases like this, someone
could just point to the prepublication post.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
CrapScientist
Harmless

Posts: 10
Registered: 6-12-2008
Member Is Offline
Mood: No Mood
|
|
In defense of stereochem and to agree with smuv up there, I'd like to also point out that as an undergrad currently enrolled in ochem I can verify
that ochem classes only really cover theory and hardly ever discuss the practical. Sure we have lab, but its basically a recipe to turn this one
thing in these other things and to quantify yields and mp/refractive index of those things we just made. That teaches us how to get around the lab
but it doesnt teach us the why of the procedure, and there is absolutely NO research involved in most undergrad organic chemistry courses. We have a
hard enough time remembering reactions and mechanisms of basic stuff, they aren't going to make us research more esoteric and complicated topics for
an undergrad course.
This is the reason why I'm starting my own hobby lab. I guess I dont process information very well when its spoon fed to me without any practical
context, so I'm buying up organic chem lab books (and vogels) and equipment and starting my own studies. Yeah its too late in the semester and I'll
probably have to take my class again, but at least now I'm taking a more proactive interest in the subject.
I dont know what stereochem's motives are and its not really that important to me. If you dont want to help him then dont help him, but its really
discouraging to newbies when people who are more knowledgable chew them out for every question they pose. This is a difficult subject and sometimes,
especially for people like me, it is a LOT easier to comprehend when we have actual people to talk about it with rather than try to learn it all on
our own.
[Edited on 7-12-2008 by CrapScientist]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I cannot believe that universities do not teach undergrad chem students how to do literature searches.
All I hear are complaints about how much journal subscriptions cost for libraries, not to mentions Chem Abstracts and online services.
So am I to understand that all of that is solely for the benefit of faculty and grad students, postdocs etc?
ACS has student memberships and low student rates for subscriptions, as an undergrad chem major I was strongly encouraged to join ACS and two journals
came with the membership (in my case JACS and JOC). I spent a great deal of time in the library searching the lit. as an undergrad. Have things
declined so very much in 40-45 years?
The whole point of studying German as an undergrad chem major was to make the chemical lit. more accesible. (I had the option of Russian as well.)
One or the other was a degree requirement. I'd had the German in high school as well, and also French in both. Chem majors were placed in courses
stressing reading comprehension rather than four-skills.
I think suspicions as to motives are unwarranted. aryl ketones are made this way (e.g., acetophenone), benzyl ketones are not (such as P2P).
[Edited on 8-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
| Quote: | Originally posted by smuv
In the US, undergrads are not taught to research, and are spoon-fed all the information they need. If I didn't have an interest in amateur chemistry,
I would have only very basic research skills. Therefore, while I think stereochem IS lazy, I do empathize with him as he is in the same boat as a lot
of undergrad chem students.
It might be handy if someone wrote a brief article in prepublication explaining the basics of chemistry research. Then, in cases like this, someone
could just point to the prepublication post. |
I disagree. I am an undergrad in the U.S. and the professors in several departments have taught us how to do proper research (perhaps my school is in
the minority), but maybe that is just augmented in my case from being on this forum. Many schools with a decent science department have some sort of
subscriptions to scientific journals, which I use when I can, but find that many of the papers listed would only be available by interlibrary loan, or
by asking nicely in references here.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
CrapScientist
Harmless

Posts: 10
Registered: 6-12-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sauron
I cannot believe that universities do not teach undergrad chem students how to do literature searches.
All I hear are complaints about how much journal subscriptions cost for libraries, not to mentions Chem Abstracts and online services.
So am I to understand that all of that is solely for the benefit of faculty and grad students, postdocs etc?
ACS has student memberships and low student rates for subscriptions, as an undergrad chem major I was strongly encouraged to join ACS and two journals
came with the membership (in my case JACS and JOC). I spent a great deal of time in the library searching the lit. as an undergrad. Have things
declined so very much in 40-45 years?
The whole point of studying German as an undergrad chem major was to make the chemical lit. more accesible. (I had the option of Russian as well.)
One or the other was a degree requirement. I'd had the German in high school as well, and also French in both. Chem majors were placed in courses
stressing reading comprehension rather than four-skills. |
1) yes things have gotten that bad. we have been trained to be dumber since birth and its quite the uphill struggle for some of us to overcome.
2) As far as chemistry goes, I've never had a class that required any research whatsoever. However, since I am a biotech student and have numerous
biology related courses, I do have to know how to use the library research center. Its pretty sweet, it searches dozens of academic search engines
which cover thousands and thousands of journals and occasionally finds me electronic copies for free. Most undergrad chem students dont need to know
this, however. Research facilities are definitely mostly for grad, post doc, and faculty.
3) You learned german just so you could read all the german research articles? That's hardcore man.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
As I said: chem major undergrads were required to learn German and/or Russian solely for the purpose of being able to read the German and Russian
chemical literature. That was not hardcore, that was routine.
If I had it to do over again I'd have opted for Russian in addition to German and French. French was not required but since my original plan was to be
a chemical patent lawyer, and I grew up in a Code Napoleon state (Louisiana) French was mandatory in pre-law. Still it has served me whel when reading
Compt.Rend. and so on. (I never did gat around to law school. I did take the LSAT and placed 98%ile nationally. Later when I was hiring patent lawyers
to work on my patents I found out how boring patent law is, and was glad I strayed from that career path.
Sic gorgeamus a los subjectatus nunc.
|
|
|
CrapScientist
Harmless

Posts: 10
Registered: 6-12-2008
Member Is Offline
Mood: No Mood
|
|
Funny, I was going to go into patent law too (the idea of getting paid a lot of money and being my own boss sounds nice) but I decided against it for
the same reasons.
Sucks they dont have language requirements in the sciences anymore. I mean I'm glad I dont have more stuff I have to take on top of what already
seems like a mountain of subjects, but I think learning another language would greatly benefit us beyond just being able to read foreign research
papers. To be honest, I switched from working on my general AA to undergrad biotech and I looked over the science curriculum requirements and NOWHERE
was there even english courses, history, or any of the normal requirements one would have to fill in any other department. I guess its too much for
science majors these days to have to learn about anything that isn't science or math, which I think is yet another shitty reminder that these
disciplines are being tailored to create good workers and not well educated people.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
IMHO, mediocre teaching at he university is no excuse for such arrogance and lazyness. Actually, it would mean you would have to do more by
yourself... And you would go no where with such attitude.
I am not really concerned with the issu of motives, obviosuly up to some limits, I prefer helping out someone that's thinking of making an illicit
substance, but is ready to do lots of learning and efforts that go beyond the simple subject of interest, hopefully that person will discover the
magic of chemistry before even succeding in making that susbstance, and will realize that the means are so much more interesting and source of
self-accomplishement than the initial motive, and will realize that it is much more pleasant to do chemistry without any legal threat flying over your
head, and that the selfishness of some people ruin the hobby of others, while a student asking to be spoonfed for his exam, even if he wants to
discover a cure for cancer or AIDS, is much less worthy of any help IMHO. Even if he does pass his exam etc, at no moment anyones help will trigger
the will of learning by himself, as he gets what he wants without that effort.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
CrapScientist
Harmless

Posts: 10
Registered: 6-12-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Klute
IMHO, mediocre teaching at he university is no excuse for such arrogance and lazyness. Actually, it would mean you would have to do more by
yourself... And you would go no where with such attitude.
I am not really concerned with the issu of motives, obviosuly up to some limits, I prefer helping out someone that's thinking of making an illicit
substance, but is ready to do lots of learning and efforts that go beyond the simple subject of interest, hopefully that person will discover the
magic of chemistry before even succeding in making that susbstance, and will realize that the means are so much more interesting and source of
self-accomplishement than the initial motive, and will realize that it is much more pleasant to do chemistry without any legal threat flying over your
head, and that the selfishness of some people ruin the hobby of others, while a student asking to be spoonfed for his exam, even if he wants to
discover a cure for cancer or AIDS, is much less worthy of any help IMHO. Even if he does pass his exam etc, at no moment anyones help will trigger
the will of learning by himself, as he gets what he wants without that effort. |
I agree for the most part, but the only real point that I'm trying to make is that it is sometimes easier for people to learn from others than by
themselves. If everyone should just learn for themselves then whats the point of having a discussion forum? Somebody can still have the desire to
learn and ask of someone else for help on the subject, its not some unwritten rule that the only way to learn anything is to keep to yourself and read
dense uninspiring tomes all day.
Another point that I was making is that modern organic chem books and courses dont teach practical methods of the reactions they cover...its just
mechanisms and theoretical "this and this reacts to give you this" without going over how its actually done in a lab setting. Maybe that's what he
was asking, I dont really know. I'm just making general points here.
[Edited on 7-12-2008 by CrapScientist]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
If it were not for this forum, I would not know about nor would have ever referred to: Vogel, orgsyn, Chem. Rev. or Org. React. These are among the
sources that I would go to first for practical information about the friedel-crafts acylation.
I go to a good school, and that is how things are. If people don't know where to start, I don't blame them for failing to do research; although, I
also understand the opposing view.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
stereochem
Harmless

Posts: 12
Registered: 29-3-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Klute
IMHO, mediocre teaching at he university is no excuse for such arrogance and lazyness. Actually, it would mean you would have to do more by
yourself... And you would go no where with such attitude.
I am not really concerned with the issu of motives, obviosuly up to some limits, I prefer helping out someone that's thinking of making an illicit
substance, but is ready to do lots of learning and efforts that go beyond the simple subject of interest, hopefully that person will discover the
magic of chemistry before even succeding in making that susbstance, and will realize that the means are so much more interesting and source of
self-accomplishement than the initial motive, and will realize that it is much more pleasant to do chemistry without any legal threat flying over your
head, and that the selfishness of some people ruin the hobby of others, while a student asking to be spoonfed for his exam, even if he wants to
discover a cure for cancer or AIDS, is much less worthy of any help IMHO. Even if he does pass his exam etc, at no moment anyones help will trigger
the will of learning by himself, as he gets what he wants without that effort. |
Before asking here I was looking in OR, and I found in vol 3 alchilation, but no acylation, in orgsyn I found acylations with acyl chlorides, but not
with anhydrides and so.
I want to apologize if I was rude.
|
|
|
CrapScientist
Harmless

Posts: 10
Registered: 6-12-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by smuv
If it were not for this forum, I would not know about nor would have ever referred to: Vogel, orgsyn, Chem. Rev. or Org. React. These are among the
sources that I would go to first for practical information about the friedel-crafts acylation.
I go to a good school, and that is how things are. If people don't know where to start, I don't blame them for failing to do research; although, I
also understand the opposing view. |
I feel dumb for asking this but, for myself and the sake of others possibly reading this thread, can you please elaborate on what those
books/references are in detail so we can also benefit from their use?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Vogels: very complete practical chemistry book, with hundred of preparations, both practical and theorical discussions, availble for free from the SM
library
OrgSyn.org: free-acces collection of peer-reviewed preparations, very complete, lots of procedures, a true reference.
Chemical reviews, Organic reactions, etc pretty similar, not all peer-reviewed, but no free acces. Members can often acces a specific preparation if
asked kindly in the ref forum.
EDIT: sorry if I got a bit carried away above, not a very good day for me. CrapScientist, I agree with what you said above, it just that Stereochem
was far from that behaviour. I agree there is a lack of real manipulations in most chemistry course, and deeply regret that. I hope wih acces to
primary litt he will be able to do some searching for himself, and ask more elaborate questions if in need.
Also: ACS home page http://pubs.acs.org/
home page of a major editor, you can use the search engine to locate an article, then ask someone with acces to retreive it for you.
Also see Wiley, Elsevier, ect simialr editors.
I think doing a large thread regrouping all this kind of information would be a good idea. i have already proposed such a thing in the ref forum, and
I think it would save a lot of time, and help alot of people accesing/researching the litterature.
There is already a free-acces journals thread which leads to alot of information.
[Edited on 8-12-2008 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Merck Index 12th Ed:
138. Friedel-Crafts Reaction C. Friedel, J. M. Crafts, Compt. Rend. 84, 1392, 1450 (1877).
The alkylation or acylation of aromatic compounds catalyzed by aluminum chloride or other Lewis acids: See Diagram
Reviews: C. C. Price, Org. React. 3, 1 (1946); G. A. Olah, Friedel-Crafts and Related Reactions, vol. 1-4 (Interscience, New York, 1963-1965); J. K.
Groves, Chem. Soc. Rev. 1, 73 (1972); H. Heaney, Comp. Org. Syn. 2, 733-752, 753-768 (1991); 3, 293-339. Aliphatic version: S. C. Eyley, ibid. 2,
707-731. Cf. Darzens-Nenitzescu Synthesis of Ketones; Haworth Phenanthrene Synthesis; Nencki Reaction.
My recollection is that Org Syn contains numerous examples of F-C acylation, and though the specific examples may all be with acyl chlorides, the
discussion and references ought to contain examples of uses of acid anhydrides.
Follow the trail of breadcrumbs.
Have you tried Google?
Have you tried the ACS Pubs search engine?
Did you read through the Org Reactions monograph or did you stop when you reckoned it was alkylations only?
What is the specific ketone that is your target?
Specific aromatic ring? Specific acid anhydride?
[Edited on 8-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
polyphosphoric acids are also claimed to work as well.
|
|
|
| Pages:
1
2 |