| Pages:
1
2 |
BobHawson
Harmless

Posts: 34
Registered: 10-12-2008
Location: USA
Member Is Offline
Mood: Regular
|
|
2-Bromo-PA into PA
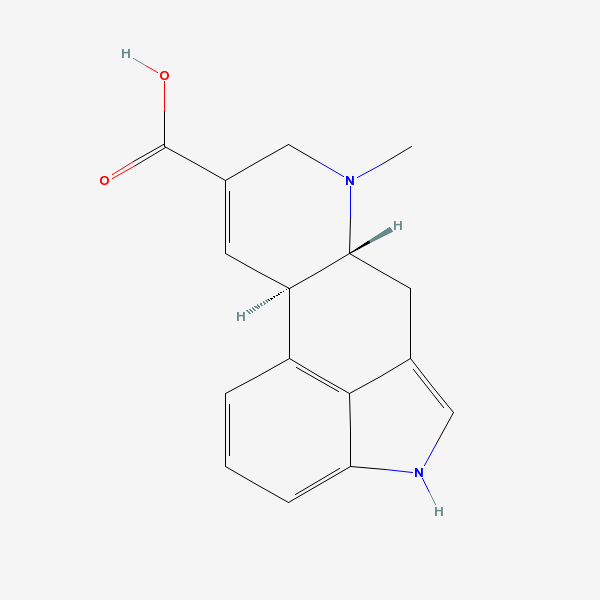
How would one convert 2-Bromo-Paspalic acid into Paspalic acid?
I've searched the literature for this, or similar reactions, but couldn't find anything.
BobHawson
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Never heard of paspallic acid, what is the structure?
Sic gorgeamus a los subjectatus nunc.
|
|
|
BobHawson
Harmless

Posts: 34
Registered: 10-12-2008
Location: USA
Member Is Offline
Mood: Regular
|
|
BobHawson
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
So much for the structure of the parent acid. So you want to dehalogenate the 2-Br derivative without affecting anything else.
Where is the 2-position on this polycyclic system? This is an a,b-unsaturated acid. If the 2-position is the beta site and the bromo derivative is
saturated then getting the Br to eliminate and form that conjugated pi bond ought to be easy. You just need to find a mild reagent (base likely) to do
the job selectively.
Google never heard of paspallic acid either.
ACS search engine returns 86 hits for 2-bromopaspallic acid all centering on marine ecology and oceanic biochemistry.
[Edited on 22-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | Originally posted by Sauron
Google never heard of paspallic acid either. |
because it is spelled PASPALIC ACID
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Well, since the very first hit on Google under that spelling is SYNTHESIS OF LYSERGIC ACID FROM PASPALIC ACID
... I am out of here and I suggest a moderator take note.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Please do not open threads without providing at least a minimum of references, unless in the Beginnings section! I will be moving this kind of threads
there anyway, so what is the point of not opening them in Beginnings in the first place? Also, use the search engine before posting.
[Edited on 22/12/2008 by Nicodem]
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
From the third post, it was clear that he wanted to fiddle with ergot alkaloids. 
Surely not for parturition purposes, good sir? 
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
BobHawson
Harmless

Posts: 34
Registered: 10-12-2008
Location: USA
Member Is Offline
Mood: Regular
|
|
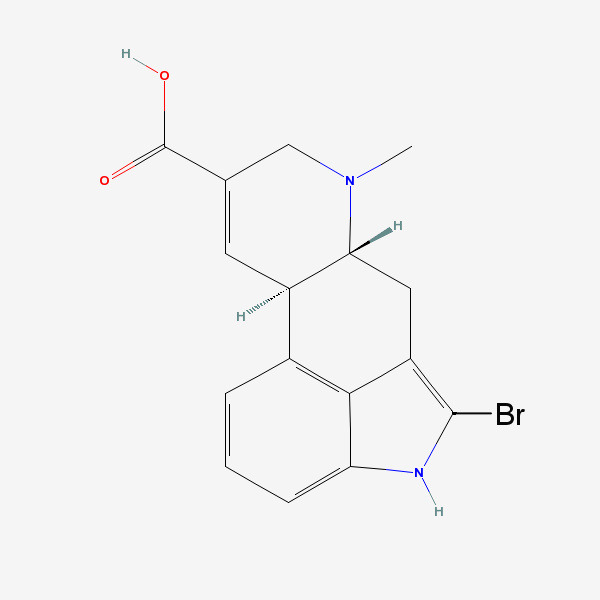
How to off the bromine atom here:
[Edited on 22-12-2008 by BobHawson]
BobHawson
|
|
|
harrydrez
Harmless

Posts: 26
Registered: 28-11-2008
Location: usa
Member Is Offline
Mood: content
|
|
| Quote: | Originally posted by sparkgap
From the third post, it was clear that he wanted to fiddle with ergot alkaloids. 
Surely not for parturition purposes, good sir? 
sparky (~_~) |
He wants to cure his migraines, funny, third post was like a neon sign screaming "LSD!"
|
|
|
Trifluoroacetic
Hazard to Others
  
Posts: 128
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
print out the image and cut off the Br with a pair of scissors and you will have what you need.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
He does seem truly lost and forlorn, doesn't he?
Try searching 2-bromoindole and seeing how to debrominate that You do not however want to reduce that double bond. You want to replace Br with H,
that' all. And that's a clue.
Sic gorgeamus a los subjectatus nunc.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Paspalic acid? It must be connected with the Paspalum genus of grasses, from Africa, which are good in pasture for grazing by livestock, but terrible
in lawns because of their seed-heads which are sticky when ripe. They are very common here in New Zealand.
So its structure is related to that of ergotamine and LSD? Perhaps it is formed on paspalum seed-heads if the ergot fungus gets at them, similarly to
rye. Both of the Ns, and two of the C atoms, are optically active.
[Edited on 23-12-08 by JohnWW]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The stereochemistry is pretty daunting, the total syntheses are daunting, the really hard part is making this stuff without autointoxicating, which is
a lab environment is pretty hazardous.
I do not think the thread author is in any real danger of getting to that point, however.
Google still barfs on 2-bromopaspalic acid, no hits at all. Is this perhaps a medicinal ergot derivative? There are lots of those used for migraines
and so on, also in obstetrics. Ergotamine tartrate being the best known, and probably most common precursor to the recreational derivative.
I was once sitting in the office of US Customs Investigations chatting with a pal there and they had just caught a fellow on a currency violation with
$25,000 cash that he failed to declare, and on him was a list of chemicals to buy in his destination (Italy). and since I am a chemist they showed me
the list. Top item: A Kg of ergotamine tartrate. I laughed out loud.
AFAIK paspalic acid is just another hydrolysis product of ergot alkaloid(s) and is produced industrially by same methods as lysergic acid, i.e.,
fermentation of appropriate fungi, Claviceps in particular.
So far I have seen nothing about 2-bromopaspalic acid anywhere, and am only looking desultorily out of curiosity as to what the thread author is on
about.
[Edited on 23-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Geez, is it cheap? If it is, you could try refluxing it with a little Potassium Hydride. That might work.
Gotta be careful with molecules like that though. Might isomerize, might react adversely to light, might accidentally become lysergic acid...Sure
does resemble it.
At any rate, such chemistry is extra-ordinarily arcane. This one is probably up to you to figure out. A few hours, days, weeks, or possibly years,
in a good research library, should give you the information you desire.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
To elucidate on my previous post, http://en.wikipedia.org/wiki/Paspalum , which lists the various species of the paspalum genus, says "Claviceps paspali is a Claviceps sac fungus
that grows on Paspalum, producing ergot alkaloids and the tremorgen paspalitrem; it causes "paspalum staggers" poisoning in cattle." That must be what
gives rise to paspalic acid in ripe paspalum seed-heads. The paspalum genus is of tropical or subtropical American origin.
The species of paspalum used for pastures in New Zealand, which is also an undesirable lawn weed because of its clumping habit and tall seed-heads, is
paspalum dilatatum; see http://www.yates.co.nz/problem-solver/problems/paspalum/ and http://www.finelawn.co.nz/index.asp?PageID=2145839048 and http://www.rnzih.org.nz/Plant_Doctor/Paspalum.htm . It can be controlled using Roundup (glyphosate). Also found in New Zealand is paspalum
vaginatum, which resembles bermudagrass but has a higher salinity tolerance, being in fact used for low-lying golf courses etc.. Another introduced
weed species in New Zealand, of no agricultural use, is mercer grass, paspalum distichum, also known as water couch or knot grass, and which requires
even stronger herbicides than Roundup for control.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
John, since the ergot fungus of interest is Claviceps paspali and the grass is paspallum, the question is which is named for what, taxonomically? But
irregardless, the connection is clear. Equally clear is that the ergot fungus is not confined to central europe as Merck Index would have us believe,
but present in Africa, parts of the Americas and possibly NZ and Australia as well.
zed, I seriously doubt that any ergot alkaloid hydrolysis product is cheap. And that is what paspalic acid is, and therefore what 2-bromopaspalic acid
is derived from. KOH is known to isomerize PA to LA and ILA and the latter is particularly undesirable. There is a patented workaround involving a PTC
with ot without a small amount of alkali metal hydroxide, as discussed ad nauseam on the usual suspect websites. The primarily lit. is in Helvetica
Chem Acta as you might expect.
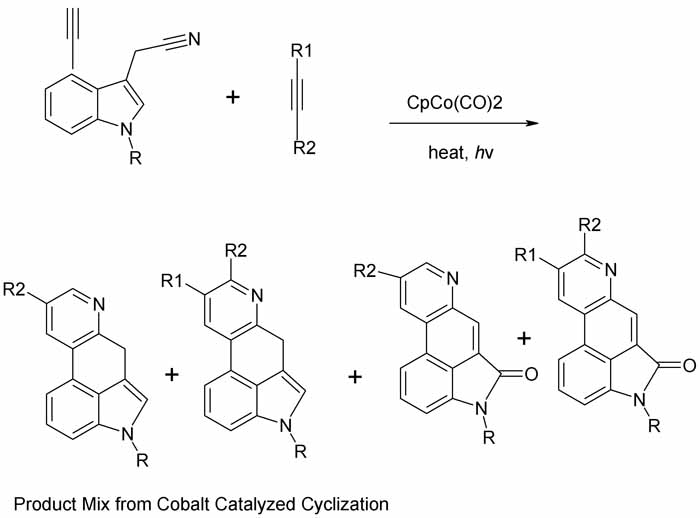
The classic Woodward total synthesis of lysergic acid is very long (16 steps). A shorter rather elegant route emerged in Synlett 1994 p 487-489
starting from 4-ethynylindole-3-acetonitrile. That compound is available from 4-bromoindole. The tetracyclic ergoline skeleton os constructed in a
single step employing a cobalt-cyclopentadienyl catalyst and a suitably substituted alkyne for 4 + 2 cyclization to a b-pyridine annelated product.
The procedure is technique intensive, yields are moderate, and the products are 4-component mixtures that require chromatographic seperation. But
still, beautiful work, and this is all chemistry and not microbiology as is usually the case. Racemic LSD tartrate was prepared in seven steps from
4-bromoindole and overall 11% yld. Still would need resolution but that can be done enzymatically (papain) since it is an amide.
Maybe this approach has been improved since 1994?
Sorry about the crappy scan, that's the way it is on my Synlett archive from References.
[Edited on 25-12-2008 by Sauron]
Attachment: p487.pdf (239kB)
This file has been downloaded 590 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Since the pdf is so muddy I have redrawn scheme 2
R = H, Me
For R1 and R2 see pdf. For catalyst see references. Looks like a cyclopentadienyl comples with a cobalt carbonyl, what fun!
The indole reactant is compound 5, the alkyne is 2, the products 6,7,8,9. 8 and 9 arise from air traces in reaction. 6 and 7 are main products due to
failure to achieve regioselectivity and tendency of R1 to hydrolyze when vicinal to N. For ring numbering and identification of rings A-D see pdf.
[Edited on 25-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
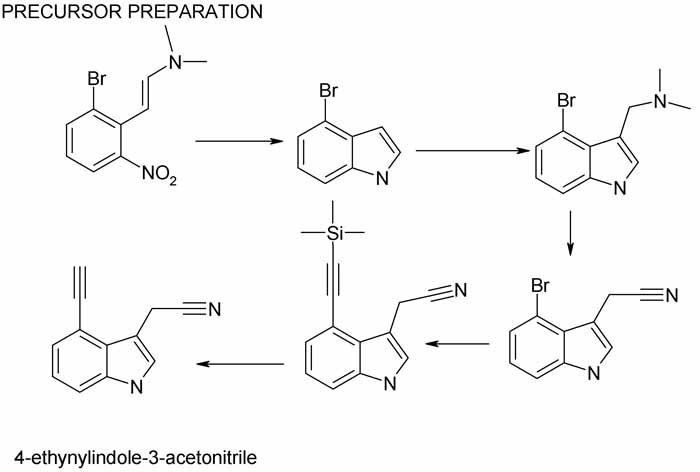
4-ethynyl-3-indoleacetonitrile may look exotic but it is readily constructed from 2-bromo-6-nitro-(2-dimethylamino)styrene.
The indole ring is formed with Fe powder, acetic acid and silica gel in benzene/cyclohexane.
Aqueous dimethylamine 40%, 37%formalin and acetic acid add at the 3-position
KCN in aqueous DMF converts the teriary amine to indole-3-acetonitrile.
Finally trimethylsilylethyne is added at the 4-bromo site and the TMS group removed with PPh3I2, PdCl2 abd 3% CuI in TEA under autogenous pressure for
18 hrs.
See scheme and references in pdf posted above. Yields are high.
[Edited on 25-12-2008 by Sauron]
[Edited on 25-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Timethylsilylethyne (TMS-acetylene) is made, per Org.Syn., from n-butylmagnesium chloride in THF reacted with acetylene gas and then the acetylene
Grignard reagent reacted with trimethylsilyl chloride.
Monograph pdf attached.
I am scratching my head opver the starting material. A beta-dimethylaminostyrene? A (2-dimethylamino)phenylethene? Any suggestions as to nomenclature
and preparation, anyone?
how about 1,2-styrenediol (styrene glycol)?
Chlorinate the 2 position, slap a dimethylamino group on there and dehydrate that benzylic hydroxyl?
Ring nitrate it and isolate the 2-nitro product, and monobrominate last.
May have to block the 4-position with a sulfonic acid to preclude dibromination, maybe do that before the nitration.
Hmmm.
[Edited on 25-12-2008 by Sauron]
Attachment: CV8P0606.pdf (157kB)
This file has been downloaded 1695 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
My 14 year-old grand daughter tells me that when she and her friends need Lysergic Acid, and her Swiss chemical broker can't get them Ergotamine,
because he is in prison again...... They simply resort to the Hendrickson-Wang Synthesis.
Embarrassingly, the entire paper is posted on that nasty Rhodium site, but it is hard to find elsewhere. Hard to find for free, at any rate.
http://www.erowid.org/archive/rhodium/chemistry/lysergic.hen...
Could my beloved little Munchkin-kiss, be headed down a perilous path?
[Edited on 25-12-2008 by zed]
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
I can't tell if your joking but if you have a 14 year old granddaughter making lysergic acid you need to enroll her in some sort of program (organic
chemistry not drug rehab).
From what I can tell lysergic acid is very delicate and atmosphere, light, moisture etc will destroy. Also from a practical standpoint i would assume
all indole based compounds are restricted.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I think zed said something very similar about the fragility of this structural class upthread.
Thanks, zed, very interesting. A decade fresher than the paper from SynLett, I guess we are creeping up on the state of the art.
Both routes, though very different, share 4-bromoindole as one of the key precursors. The 1994 prep is like falling off a log, while the 2004 prep
uses TFA and thallium trifluoroacetate. TFA is merely aggressive and unpleasant even in a hood, thallium salts are megatoxic. I will need more time
before I can comment further about the relative merits of these two routes. But very interesting indeed.
Oh, by the by:
the thread author was asking about 2-bromopaspalic acid.
See the Merck Index for bromolysergide. That is bromo-LSD. Inhibits serotonin but devoid of hallucinatory effects.
My guess is that a bulky Br vicinal to the indole N lone pair blocks interaction with receptor sites for those effects.
So I suppose thread author wants to remove Br at C2 so he can convert to LA. No surprise.
----------------
Thallium triflate is also horribly expensive. Over $50/g and 30 g are used in prep in the Org.Lett. paper.
The indole ring is readily built from 2-nitrotoluene using ethyl oxalate. See Org.Syn. The resulting 2-ethylindole carboxylate is readily hydrolyzed
and decarboxylated. What is wrong with doing same procedure to 6-bromo-2-nitrotoluene? Or 6-iodo-2-nitrotoluene?
[Edited on 26-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
So, can't he make the Grignard reagent and hydrolize it? Or is paspalic acid too delicate? And is there a commercially available 2-Br-paspalic acid?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The answer to all is "Who knows?"
I have yet to find any reference ro 2-bromopaspalic acid.
I do not have a clue how fragile it might be. I would expect the Grignard to potentially react with the carboxylic acid site though.
--------
There are lots of indole preps in the lit. and surely several will allow starting with a ring Br that will survive under reaction conditions.
The 1994 SynLett paper uses Fe powder and AcOH in benzene/cyclohexane to cyclize 6-bromo-2-nitro-(2-dimethylamini)styrene to 4-bromoindole.
There are half a dozen generally similar reactions in Org.Syn.
Back in the Melatonin thread I looked at a few of these.
It's just a matter of jumping back into indole chemistry and finding ane answers. 4-Bromoindole is commercially available but outrageously expensive.
Our target is 4-bromoindole.
Here's an Org.Syn prep to a 4-chloro-5-azaindole-2-carbomethyl ester.
They start with 2-chloropyridine-3-carboxaldehyde. I would just substitute 2-bromobenzaldehyde, or 2-iodobenzaldehyde and follow their sequence from
there.
The aldehyde is reacted with ethyl azidoacetate (from ethylbromoacetate and sodium azide) which is readily cyclized to the indole, and I know that the
methyl ester is easy to hydrolyze and the acid group at 2 position decarboxylates. Voila. 4-haloindole. No TFA, no thallium (III) triflate.
[Edited on 26-12-2008 by Sauron]
[Edited on 26-12-2008 by Sauron]
Attachment: V84P0262.pdf (139kB)
This file has been downloaded 916 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
| Pages:
1
2 |